Practical Biology
A collection of experiments that demonstrate biological concepts and processes.


Observing earthworm locomotion

Practical Work for Learning

Published experiments
Investigating an enzyme-controlled reaction: catalase and hydrogen peroxide concentration, class practical or demonstration.
Hydrogen peroxide ( H 2 O 2 ) is a by-product of respiration and is made in all living cells. Hydrogen peroxide is harmful and must be removed as soon as it is produced in the cell. Cells make the enzyme catalase to remove hydrogen peroxide.
This investigation looks at the rate of oxygen production by the catalase in pureed potato as the concentration of hydrogen peroxide varies. The oxygen produced in 30 seconds is collected over water. Then the rate of reaction is calculated.
Lesson organisation
You could run this investigation as a demonstration at two different concentrations, or with groups of students each working with a different concentration of hydrogen peroxide. Individual students may then have time to gather repeat data. Groups of three could work to collect results for 5 different concentrations and rotate the roles of apparatus manipulator, result reader and scribe. Collating and comparing class results allows students to look for anomalous and inconsistent data.

Apparatus and Chemicals
For each group of students:.
Pneumatic trough/ plastic bowl/ access to suitable sink of water
Conical flask, 100 cm 3 , 2
Syringe (2 cm 3 ) to fit the second hole of the rubber bung, 1
Measuring cylinder, 100 cm 3 , 1
Measuring cylinder, 50 cm 3 , 1
Clamp stand, boss and clamp, 2
Stopclock/ stopwatch
For the class – set up by technician/ teacher:
Hydrogen peroxide, range of concentrations, 10 vol, 15 vol, 20 vol, 25 vol, and 30 vol, 2 cm 3 per group of each concentration ( Note 1 )
Pureed potato, fresh, in beaker with syringe to measure at least 20 cm 3 , 20 cm 3 per group per concentration of peroxide investigated ( Note 2 )
Rubber bung, 2-holed, to fit 100 cm 3 conical flasks – delivery tube in one hole (connected to 50 cm rubber tubing)
Health & Safety and Technical notes
Wear eye protection and cover clothing when handling hydrogen peroxide. Wash splashes of pureed potato or peroxide off the skin immediately. Be aware of pressure building up if reaction vessels become blocked. Take care inserting the bung in the conical flask – it needs to be a tight fit, so push and twist the bung in with care.
Read our standard health & safety guidance
1 Hydrogen peroxide: (See CLEAPSS Hazcard) Solutions less than 18 vol are LOW HAZARD. Solutions at concentrations of 18-28 vol are IRRITANT. Take care when removing the cap of the reagent bottle, as gas pressure may have built up inside. Dilute immediately before use and put in a clean brown bottle, because dilution also dilutes the decomposition inhibitor. Keep in brown bottles because hydrogen peroxide degrades faster in the light. Discard all unused solution. Do not return solution to stock bottles, because contaminants may cause decomposition and the stock bottle may explode after a time.
2 Pureed potato may irritate some people’s skin. Make fresh for each lesson, because catalase activity reduces noticeably over 2/3 hours. You might need to add water to make it less viscous and easier to use. Discs of potato react too slowly.
3 If the bubbles from the rubber tubing are too big, insert a glass pipette or glass tubing into the end of the rubber tube.
SAFETY: Wear eye protection and protect clothing from hydrogen peroxide. Rinse splashes of peroxide and pureed potato off the skin as quickly as possible.
Preparation
a Make just enough diluted hydrogen peroxide just before the lesson. Set out in brown bottles ( Note 1 ).
b Make pureed potato fresh for each lesson ( Note 2 ).
c Make up 2-holed bungs as described in apparatus list and in diagram.
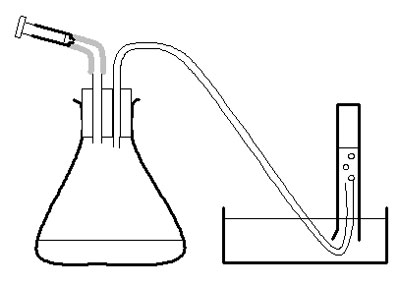
Investigation
d Use the large syringe to measure 20 cm 3 pureed potato into the conical flask.
e Put the bung securely in the flask – twist and push carefully.
f Half-fill the trough, bowl or sink with water.
g Fill the 50 cm 3 measuring cylinder with water. Invert it over the trough of water, with the open end under the surface of the water in the bowl, and with the end of the rubber tubing in the measuring cylinder. Clamp in place.
h Measure 2 cm 3 of hydrogen peroxide into the 2 cm 3 syringe. Put the syringe in place in the bung of the flask, but do not push the plunger straight away.
i Check the rubber tube is safely in the measuring cylinder. Push the plunger on the syringe and immediately start the stopclock.
j After 30 seconds, note the volume of oxygen in the measuring cylinder in a suitable table of results. ( Note 3 .)
k Empty and rinse the conical flask. Measure another 20 cm 3 pureed potato into it. Reassemble the apparatus, refill the measuring cylinder, and repeat from g to j with another concentration of hydrogen peroxide. Use a 100 cm 3 measuring cylinder for concentrations of hydrogen peroxide over 20 vol.
l Calculate the rate of oxygen production in cm 3 /s.
m Plot a graph of rate of oxygen production against concentration of hydrogen peroxide.
Teaching notes
Note the units for measuring the concentration of hydrogen peroxide – these are not SI units. 10 vol hydrogen peroxide will produce 10 cm 3 of oxygen from every cm 3 that decomposes.( Note 1 .)
In this procedure, 2 cm 3 of 10 vol hydrogen peroxide will release 20 cm 3 of oxygen if the reaction goes to completion. 2 cm 3 of liquid are added to the flask each time. So if the apparatus is free of leaks, 22 cm 3 of water should be displaced in the measuring cylinder with 10 vol hydrogen peroxide. Oxygen is soluble in water, but dissolves only slowly in water at normal room temperatures.
Use this information as a check on the practical set-up. Values below 22 cm 3 show that oxygen has escaped, or the hydrogen peroxide has not fully reacted, or the hydrogen peroxide concentration is not as expected. Ask students to explain how values over 22 cm 3 could happen.
An error of ± 0.05 cm 3 in measuring out 30 vol hydrogen peroxide could make an error of ± 1.5 cm 3 in oxygen production.
Liver also contains catalase, but handling offal is more controversial with students and introduces a greater hygiene risk. Also, the reaction is so vigorous that bubbles of mixture can carry pieces of liver into the delivery tube.
If collecting the gas over water is complicated, and you have access to a 100 cm 3 gas syringe, you could collect the gas in that instead. Be sure to clamp the gas syringe securely but carefully.
The reaction is exothermic. Students may notice the heat if they put their hands on the conical flask. How will this affect the results?
Health and safety checked, September 2008
http://www.saps.org.uk/secondary/teaching-resources/293-student-sheet-24-microscale-investigations-with-catalase Microscale investigations with catalase – which has been transcribed onto this site at Investigating catalase activity in different plant tissues.
(Website accessed October 2011)

COMMENTS
Feb 3, 2010 · Enzyme concentration affects the rate of reaction; The higher the enzyme concentration in a reaction mixture, the greater the number of active sites available and the greater the likelihood of enzyme-substrate complex formation; As long as there is sufficient substrate available, the initial rate of reaction increases linearly with enzyme ...
It is always best to check the enzyme activity in advance. In the ICT support there is a datalogging sheet on monitoring an enzyme-catalysed reaction. The Core Practical requires investigation of enzyme and substrate concentration. Having completed the practical investigating enzyme concentration, students can plan how to investigate substrate
Oct 19, 2022 · Substrate Concentration. In the presence of a given amount of enzyme, the rate of an enzymatic reaction increases as the substrate concentration increases until a limiting rate is reached, after which further increase in the substrate concentration produces no significant change in the reaction rate (part (a) of Figure \(\PageIndex{1}\)).
pH, enzyme concentration and substrate concentration. The effect of each of these factors can be determined by changing one variable only , and observing its effect on the rate of reaction. In this practical, we will determine the effect of enzyme concentration on the rate of protein breakdown by the enzyme Trypsin . Equipment
Oct 16, 2024 · Preparation of a starch solution of known concentration (stock solution), from which a range of concentrations are made using serial dilutions (method outlined in diagram below) Following calibration and switching on the red filter (to maximise the percentage transmission or absorbance), the colourimeter is used to measure the percentage ...
Jan 10, 2012 · the blue-black color will be seen for a longer time. You can assess the relative enzyme activity as follows: Iodine test for starch Amount of starch remaining Enzyme activity level Dark blue-black All None (0) Blue Most Low (1) Light brown Some Moderate (2) Gold None High (3) Part 1: Effect of Enzyme Concentration 1. Label five test tubes 1-5 ...
Protease enzyme solution - 1% trypsin enzyme solution 2% semi-skimmed milk Pipettes Tongs Method Factor 1 - the effect of temperature 1. Prepare the 5 water baths and monitor their temperatures using thermometers. 2. Use a pipette to add 2 cm³ of trypsin solution to 5 test tubes and place one in each water bath. 3.
Hydrogen peroxide is harmful and must be removed as soon as it is produced in the cell. Cells make the enzyme catalase to remove hydrogen peroxide. This investigation looks at the rate of oxygen production by the catalase in pureed potato as the concentration of hydrogen peroxide varies. The oxygen produced in 30 seconds is collected over water.
measured because the rate of an enzyme-controlled reaction is high , this is because enzymes act as biological catalysts, so the concentration of reactants changes rapidly. The initial rate is the only point during the reaction when concentration of reactants and products is known . Equipment Milk powder solution
Catalase is found in high concentration in raw potatoes. Use the method below to carry out an investigation into the effect of enzyme of substrate concentration on enzyme activity . Apparatus and reagents . Freshly cut potato cylinders . Pestle and mortar . Specimen tubes/test tubes . Stock solution of hydrogen peroxide . Filter paper discs ...