

Driving innovation
In clinical research, in clinical research.

At the forefront of drug evolution
-min.jpg)
We are Richmond Pharmacology.
Centre of excellence., work with us.

Meet the experts
-min.jpg)
Richmond Pharmacology has a team of experts with extensive knowledge of clinical research trials.
Careers at Richmond

We are looking for people who love what they do, strive for excellence and who want to make a difference.
.jpg)
Learn more about Richmond Pharmacology by viewing some of our videos.
Latest news
Richmond hosts cpr training for restart a heart day, richmond ceo presents landmark data on repeat-dose gene editing, pulse virtual: respiratory, cvd, diabetes and nutrition.

Knowledge Base
Connect with us.


- Central Middlesex Hospital
- testimonials
- trial design
- subject recruitment
- clinical research
- project management
- data management
- medical writing
- computer systems validation (CSV) and IT
- quality services
- adaptive studies
- bioavailability and bioequivalence
- bridging trials
- drug-drug interactions
- first-in-human healthy volunteer trials – SAD & MAD
- first-in-human patient trials
- food effect
- phase 2 patient studies
- thorough QT trials
- analysis of drug concentrations
- microdose trials
- polysomnography
- precision ECG
- cardiovascular
- central nervous system
- dermatological
- gastrointestinal
- haematological
- respiratory
- biologicals and biosimilars
- radiolabelled
- small molecules
- publications
Hammersmith Medicines Research Cumberland Avenue London NW10 7EW
For enquiry 020 8961 4130
Specialists in clinical pharmacology
Phase 1 and early phase 2 trials
Over 900 clinical trials completed since 1993 complete an online enquiry form
For pharmaceutical or biotechnology companies worldwide complete an online enquiry form
Fully integrated services from study design to final report complete an online enquiry form
- Specialist services read more
- Want to volunteer at HMR? find out more
- Careers working at HMR
- Corporate view more

Facilities and services
- We specialise in early clinical trials of study drugs for pharmaceutical and biotechnology companies worldwide.
- Founded in 1993, HMR is now the biggest CRO of its kind in the UK and one of the biggest in Europe.
- We have 270 staff, including a resident 24/7 resuscitation team, extensive purpose-built facilities, 145 beds, and on-site pharmacy, radiopharmacy, and laboratory.
- We can provide a full service from design to clinical study report, all in one building in London, UK.
- Our level of service and commitment is the same for all companies, regardless of size.
- HMR has: MHRA Phase 1 Accreditation for FIH trials; Manufacturing and Import Licence for study drugs [MIA(IMP)]; Home Office licence for all controlled drug substances; and Environment Agency licence for trials of radiolabelled study drugs.
- Our Quality System is compliant with ISO 9001, our Laboratory with ISO 17025, and our Information Security Management System with ISO 27001.
- We’ve won three Queen’s Awards for Exports or Enterprise, the most prestigious of UK business awards.

Our experience
- We have completed >900 trials for pharmaceutical and biotechnology companies from 23 countries worldwide, including all 15 major companies by revenue, and >200 small-to-medium sized or virtual companies.
- We can do almost all types of trial, from simple bioequivalent to complex trials, such as endoscopy, sleep, radiopharmacy, bridging, QTc, and PET, fMRI or CT imaging.
- Many of our trials are first-in-human (FIH) and proof-of-concept in design and range from small to large and complex trials with adaptive protocol and several parts.
- Most of the study drugs are small molecules, but we also do many trials of biological products, such as recombinant proteins, monoclonal antibodies, vaccines, and small interfering RNA molecules.
- Most of our trials are in healthy subjects, but many include patients with the target disease; some trials involve only patients.
- We have a large database of healthy subjects of all ages and ethnicities, and many types of patient.
- We collaborate with London universities, hospitals, and general practices for trials requiring patients.

Why choose HMR?
- We don’t have a business development team. We rely solely on reputation, recommendations, record, and repeat business, a unique testament to the quality of our services.
- The UK is a good place to do clinical trials: the regulatory system is rigorous, efficient, and supportive of clinical pharmacology and sponsors and can help sponsors to plan their FIH trials.
- The MHRA and FDA inspectors have never reported any important GCP or GMP findings.
- Our pharmacy prepares and QP releases all types of dose. Our laboratory provides rapid turnaround of safety samples, and a wide range of biomarkers, such as flow cytometry, cytokine, coagulation, platelet aggregation, and PCR tests.
- We developed TOPS, a database to register participants in UK phase 1 trials; use is now mandatory for regulatory approval of phase 1 trials.
- We PCR test all subjects for SARS-Cov-2, and admit only negative subjects. We PCR test all front-line staff regularly. So far we’ve kept the virus out of our trials.
- Together with sponsors, we publish results of our trials in peer-reviewed journals.
Applications are open for the Clinical Research Training Fellowship programme with the MRC
The Medical Research Council (MRC) Medicines Development Fellowship Scheme is offering funding for 4 Clinical Research...
HMR’s Environmental Management System receives ISO 14001 certification
On 21 May 2024, HMR’s Environmental Management System received ISO 14001 certification. This is one of a number of...
HMR will take part in the MRC Medicines Development Fellowship Programme
HMR is pleased to announce that it will take part in the Medical Research Council (MRC) Medicines Development Fellowship...
HMR offers Biomedical Scientist Apprenticeships
HMR offers Biomedical Scientist apprenticeships with a BSc (Hons) in Applied Bioscience from the University of...
HMR achieves ISO 27001 information security certification
HMR attained ISO 27001 information security certification in November 2021. This further builds on our Cyber Essentials...
HMR data expertise contributes to improved NHS bowel cancer screening
Our comprehensive Statistics and Data Management service has contributed to a pioneering, improved, bowel cancer...
Privacy Overview

MAC Clinical Research is a full-service, global, pharmaceutical contract research organization (CRO), with science at the heart of everything we do. Read More…
- MAC Leadership
- Our History
- Working Together
- Corporate Social Responsibility
The Home of Clinical Trials
Effective solutions to successfully deliver your clinical trials.

Clinical Research Services
- Project Management
- Clinical Operations
- Clinical Trial Feasibility
- Pharmacovigilance
- Regulatory Affairs
- Vendor Management
- Digital Solutions
- Medical Monitoring
Site & Patient Services
- Participant Recruitment
- Participant Retention
- Global Investigator Sites
Sleep Laboratory
Laboratory services.
- Early Phase Unit
- Psychedelic Testing Suites
- Dedicated Research Sites
- Memory Assessment Research Clinics
Pharmaceutical Services
- Nationwide Logistical Coverage

Methods & Technology
MAC conduct clinical trials for most of the worlds leading pharma and biotechs. Read More…
Scientific Solutions
We offer an array of scientific solutions to support any clinical trial. Read More…
- Human Models of Disease
- Clinical Assessments and Procedures
- Sleep Assessments
- Psychedelics
- Training Services
- Quality Assurance
- Adaptive Trials
- Remote Clinical Trial Support

Strategic Consulting
MAC’s flexible strategic consulting can support your project at any stage. Read More…

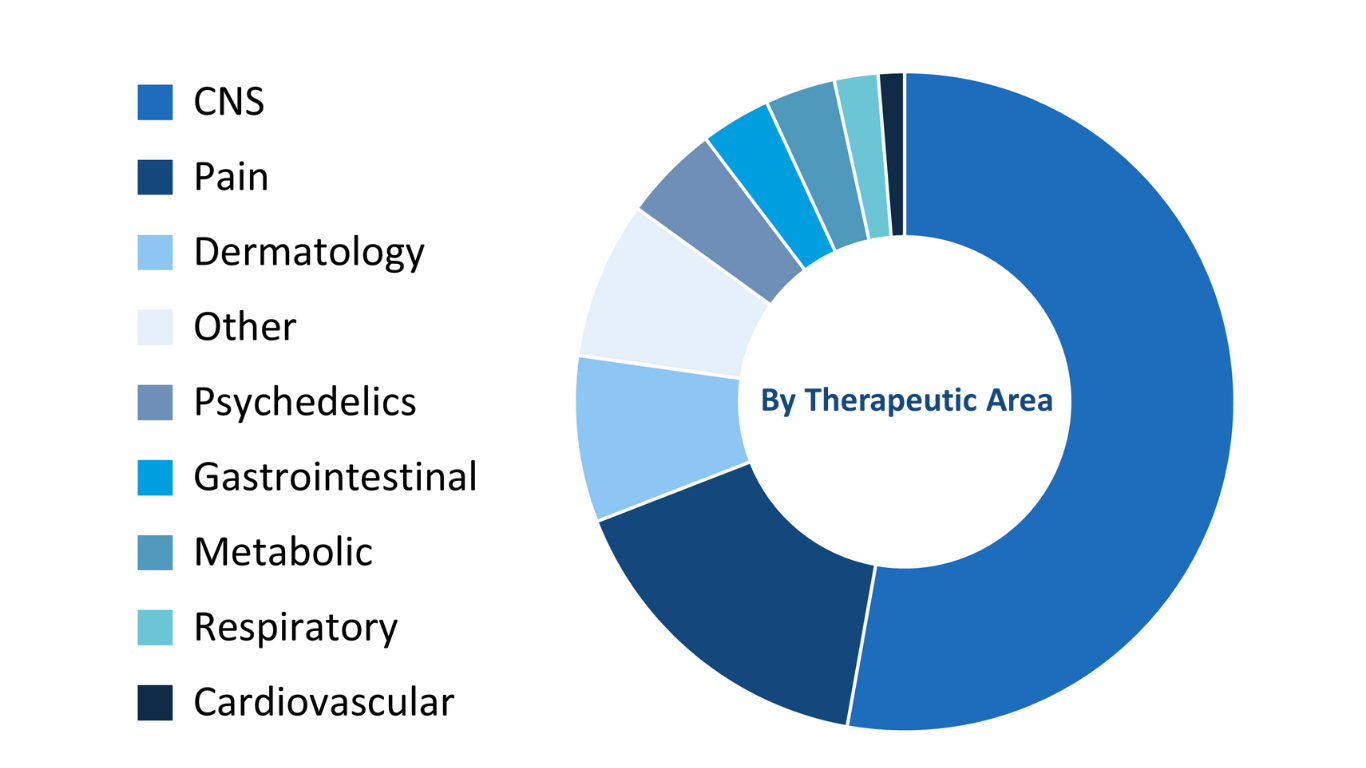
Therapeutic Expertise
Therapeutic areas.
- Case Studies
- Timeline Recovery
- Meetings & Conferences
- Resource Library
Make a Difference
- Participate In A Clinical Trial

Bespoke CRO Solutions
Mac is a full service, flexible, global contract research organisation, trusted study delivery, with mac’s fully owned dedicated research sites, mac envision® patient engagement, specialist services | proven process, phase 1 accredited unit, neuroscience centre of excellence, extensive experience | broad range of therapeutic areas, full service | biometrics solutions, bioanalytical and pathology services, delivering for you | gmp manufacturing, polysomnography expertise | state-of-the-art facility.
Contract Research Organisation
Dedicated Research Centres
Clinical Design & Development
Clinical Research Innovation
MAC Clinical Research is one of Europe’s largest contract research organisations (CRO) and has headquarters in the UK. Owning a network of Dedicated Research Sites providing extensive reach, MAC also has offices within the EU and the US. Read more …

Latest Clinical Research News
Comparative evaluation of swabbing sites for Omicron variant detection in PCR testing
We are pleased to share a recent publication in conjunction with the UK Health Security Agency (UKHSA) and insights from

Did you know MAC has eight UK Phase 1 sites?
MAC Clinical Research’s Manchester site is an MHRA audited and accredited Phase I centre located within and supported by the

Maintaining Sustainability and Inclusion Through Vendor Diversity
As well as working towards being a more sustainable company, there is a big drive for us to pass this
Privacy Overview
Let's create what's next

De-risking clinical development of precision medicines in oncology
Clinical strategies to optimise software as a medical device (samd) for treating mental health, embracing a blended operating model.
Maximising efficiency and flexibility with custom outsourcing
A multifaceted risk factor: Addressing obesity's impact across the disease spectrum
Considerations for clinical trial design focused on obesity treatments.
Demystifying EU CTR, MDR and IVDR
Submission strategies for clinical trials involving medical devices and in vitro diagnostic devices.
Applying AI to manage the risks and costs of postmarketing requirements
Cassandra offers up to 99% accuracy predicting the need for PMRs
The triad of trust: Navigating real-world healthcare data integration
Uncover how reshaping data and integration strategies can empower your organisation.
Featured Solutions
Blended solutions
Bespoke, seamless solutions to meet unique sponsor challenges.
Outcome Measures
A multi-faceted approach to identifying, selecting, and implementing evidence-based measures that matter to patients.
ICON provides full service outsourcing and flexible support for biotech specific needs such as due diligence and asset valuation.
Cardiac Safety Solutions
End-to-end cardiac safety solutions, including ECG, event monitoring, BPM, long-term Holter monitoring, ECHO and MUGA studies.
Early Clinical and Bioanalytical Solutions
Innovative early clinical solutions that will advance your drug development strategy.
Site & Patient Solutions
Transforming recruitment through patient-centric trials and real-world, real-time data.
Market Access
Expertise in mission-critical pricing, market access, and reimbursement.
Specialty Laboratory Solutions
Supporting precision medicine programs across all phases of drug diagnostic co-development.
ICON overview
ICON is expanding the possibilities of clinical research for customers, partners, and patients.
Technology solutions
ICON’s technology solutions are focused on the factors most critical to our customers.
ICON is harnessing AI with pragmatic, impactful, award winning solutions for a healthier world.
Unlocking global strategies with proactive regulatory translation management
15 November 2024. Register today.
“S” stands for systematic: Demystifying the systematic literature review
5 December, 2024. Register today.
Dosimetry in clinical trials
Watch the recording
ICON Insights
- 03 Dec 2024
The importance of communication in patient-centred clinical trials
- 27 Nov 2024
Obesity-related liver conditions: Understanding treatment options
Designing clinical trials for a complex disease: Pediatric obesity
- 18 Nov 2024
The importance of a robust clinical outcome assessment strategy in your clinical trial
- 15 Nov 2024
How an eCOA library can reduce your study start up timelines
- 14 Nov 2024
A dynamic market: Trends transforming the future of obesity drug development
- 08 Nov 2024
Submission strategies for combined studies in the EU
- 25 Oct 2024
Maximising clinical trial success with integrated language services
What’s happening in icon.
- Cell and Gene Therapies
- Early Clinical
- Medical Device
- Rare & Orphan Diseases
- Real World Evidence
- Site & Patient Recruitment
- Strategic Solutions
- Regulatory Intelligence
Diagnostics
- Search diagnostic tests
- Rapid Diagnostics Centre
- Rapid diagnostics services
- Urology clinic
- One-stop breast clinic
- Gynaecology clinic
- Dermatology clinic
- Health Assessments
- Conditions we treat
- Back to main menu
Treatments & experts
- Find an expert
- Find a treatment
Information for patients
- Treatment preparation
- Your stay with us
- Your care team
- International patients
- Your feedback
- About The London Clinic
- Our Charity
- Our heritage
- How we innovate
- Meet the board
- For professionals
The London Clinic Research Centre
Established in 2011, The Clinical Research Centre (CRC) is the onsite Clinical Trials department at The London Clinic and is dedicated to researching the latest treatments.

The CRC is responsible for managing and running clinical trials, as well as other forms of research taking place at The London Clinic.
Clinical trials are research studies that people take part in to help figure out if a medicine, surgical procedure or behavioural intervention can benefit patients' lives and improve their health.
Over the past two years, the CRC has contributed to clinical research studies in the fields of:
- Sickle Cell Disease
- Parkinson’s Disease
- Chronic obstructive pulmonary disease (COPD)
- Haematology
Performing over 200 visits and supporting other organisations through provision of specialist services.
The London Clinic’s aim is to ensure our patients have access to a wide range of treatment choices, including new therapies that may not be available anywhere else.
The London Clinic is more than a hospital, and we invest in improving patients' lives beyond treatment.
The Clinical Research Centre strives to help bring patients the newest, most innovative treatments.
The CRC also provides a range of services to leading clinical research organisations. We work closely with the BARTS Hospital, Imperial University, AstraZeneca, Ablative solutions, ReCor Medical and Queen Anne Street Medical Centre.

Clinical trials at The London Clinic
Our primary clinical trial studies include:
We have four trials currently working with new devices on the market to establish their efficiency with lowering blood pressure of patients that have exhausted all other avenues to stabilise their blood pressure.
A randomised study to evaluate the use of a certain medication that is used to treat asthma and COPD.
Three studies using DAT scans to monitor the efficacy of Parkinson’s disease treatment.
Meet our Clinical Research Centre team
Elizabeth white, clinical trial manager.
Elizabeth joined the Clinical Research Centre as Clinical Trial Manager in 2022, with over 20 years’ experience working in the field of clinical research in various capacities across the NHS, academia and private organisations.
She is responsible for the day-to-day management of clinical research at The London Clinic, from initial feasibility assessment through to close down and archiving.
Elizabeth is looking forward to expanding the department and the range of research studies on offer, whilst always considering the needs of The London Clinic's patients, the specialties and interests of its employees, in line with our values and strategy. She focuses on having The London Clinic at the forefront of exploring potential new treatments to improve patient care.
If you would like to get in touch with our Clinical Research Centre, email us today.

IMAGES
COMMENTS
Clinicology - an FGK company is a bespoke CRO based near London, UK for Phase I-IV CTIMP and medical device studies. Our advantages are our speed and flexibility, with rapid project startup and team deployment with our network across Europe and the U...
We are the UK's leading clinical research organisation, successfully conducting over 300 early phase studies since 2001. Speak to our team of experts today.
London's Leading Clinical Research Organisation (CRO). We have carried out over 650 successful trials since 1993. Clinical trials of study drugs for pharmaceutical and biotechnology companies worldwide.
MAC Clinical Research is a full-service, global, pharmaceutical contract research organization (CRO), with science at the heart of everything we do. Effective solutions to successfully deliver your clinical trials. MAC conduct clinical trials for most of the worlds leading pharma and biotechs.
ICON is the world's leading clinical research organisation, providing outsourced clincal development and commercialisation services to the pharmaceutical, biotechnology and medical device industries.
The London Clinic is more than a hospital, and we invest in improving patients' lives beyond treatment. The Clinical Research Centre strives to help bring patients the newest, most innovative treatments. The CRC also provides a range of services to leading clinical research organisations.