An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Prevention of Type 2 Diabetes by Lifestyle Changes: A Systematic Review and Meta-Analysis
Matti uusitupa, tauseef a khan, effie viguiliouk, hana kahleova, angela a rivellese, kjeld hermansen, andreas pfeiffer, anastasia thanopoulou, jordi salas-salvadó, ursula schwab, john l sievenpiper.
- Author information
- Article notes
- Copyright and License information
Correspondence: [email protected] ; Tel.: +358-400-615661
Received 2019 Sep 3; Accepted 2019 Oct 18; Collection date 2019 Nov.
Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/ ).
Prevention of type 2 diabetes (T2D) is a great challenge worldwide. The aim of this evidence synthesis was to summarize the available evidence in order to update the European Association for the Study of Diabetes (EASD) clinical practice guidelines for nutrition therapy. We conducted a systematic review and, where appropriate, meta-analyses of randomized controlled trials (RCTs) carried out in people with impaired glucose tolerance (IGT) (six studies) or dysmetabolism (one study) to answer the following questions: What is the evidence that T2D is preventable by lifestyle changes? What is the optimal diet (with a particular focus on diet quality) for prevention, and does the prevention of T2D result in a lower risk of late complications of T2D? The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was applied to assess the certainty of the trial evidence. Altogether seven RCTs (N = 4090) fulfilled the eligibility criteria and were included in the meta-analysis. The diagnosis of incident diabetes was based on an oral glucose tolerance test (OGTT). The overall risk reduction of T2D by the lifestyle interventions was 0.53 (95% CI 0.41; 0.67). Most of the trials aimed to reduce weight, increase physical activity, and apply a diet relatively low in saturated fat and high in fiber. The PREDIMED trial that did not meet eligibility criteria for inclusion in the meta-analysis was used in the final assessment of diet quality. We conclude that T2D is preventable by changing lifestyle and the risk reduction is sustained for many years after the active intervention (high certainty of evidence). Healthy dietary changes based on the current recommendations and the Mediterranean dietary pattern can be recommended for the long-term prevention of diabetes. There is limited or insufficient data to show that prevention of T2D by lifestyle changes results in a lower risk of cardiovascular and microvascular complications.
Keywords: prevention, type 2 diabetes, diet, lifestyles, complications
1. Introduction
Both the prevalence and incidence of type 2 diabetes (T2D) are increasing rapidly worldwide. Worldwide, in 2017, approximately 425 million people had diabetes. This figure may rise to 629 million by 2045. However, the figures for different European countries are not as dramatic as the figures in America and in many low- and middle-income countries. In Europe, the prevalence of T2D is also increasing in parallel to the obesity epidemic. In 2017, the number of patients with diabetes in Europe was 66 million (prevalence 9.1%) and it is estimated to be 81 million by 2045. [ 1 , 2 ]. T2D is a potent risk factor for cardiovascular diseases, but also for blindness, renal failure, and lower limb amputation, decreasing the quality of life of people affected. The burden of diabetes is not only a public health issue, but it also has marked economic consequences. More specifically, the expenses for the treatment of diabetes are increasing mostly due to its long-term complications but also modern drug treatment options [ 3 ]. Furthermore, bariatric surgery is becoming more popular for markedly obese patients with T2D due to its significant beneficial effects on metabolic control, long-term complications, and prognosis of T2D [ 4 , 5 ].
The interest in preventing diabetes through lifestyle changes was already present in the 1980s [ 6 ], and the opportunity to prevent T2D through lifestyle changes was re-emphasized in the 2004 recommendations of the Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD) [ 7 ]. Since then, a number of randomized controlled trials (RCTs) have been published that show that T2D is preventable, or its onset can be markedly postponed, by increasing physical activity, reducing weight, and changing dietary habits.
To update the evidence for the EASD clinical practice guidelines for nutrition therapy, we conducted a systematic review and, where appropriate, meta-analyses of the available randomized controlled trials assessing lifestyle interventions in the prevention of T2D with the aim of answering the following questions:
(a) What is the evidence that T2D is preventable by lifestyle changes in adults with impaired glucose tolerance (IGT) and (b) what are the long-term results on the prevention of T2D?
What is the evidence that the lifestyle changes aimed to prevent T2D also modify the risk of cardiovascular disease and microvascular complications in people with IGT?
What is the optimal dietary composition for the prevention of T2D in people with IGT?
A systematic review and meta-analysis of randomized controlled trials (RCTs) was conducted to assess the role of lifestyle changes on the prevention of T2D using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach. In addition, we discuss the lifestyle including dietary changes that have been successfully used for the prevention of T2D and summarize the long-term follow-up results after the active intervention periods from the major T2D prevention trials on the incidence of T2D and micro- and macrovascular diseases, and finally make the conclusions regarding the three study questions.
We attempt to answer these three questions in turn, summarizing the evidence following by making conclusions at the end of the paper.
2. Evidence That T2D Is Preventable by Changing Lifestyles
A systematic review and meta-analysis of randomized controlled trials (RCTs) was conducted to assess the role of lifestyle changes on the prevention of T2D using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.
3.1. Search Strategy and Study Selection
We conducted our systematic review and meta-analysis according to the Cochrane Handbook for Systematic Reviews of Interventions [ 8 ], and reported the results according to the PRISMA guidelines ( www.prisma-statement.org ). We conducted standard literature searches of PubMed (MEDLINE), EMBASE, and Cochrane Library through 21 June 2019 to identify both original RCTs and recent systematic reviews [ 9 , 10 , 11 , 12 ] that have examined the association of lifestyle intervention with T2D. The following key words were used in selecting original RCTs for this search: type 2 diabetes, RCT, prevention, systematic reviews, impaired glucose tolerance (IGT), diet, dietary pattern, physical activity, and lifestyle. We supplemented the systematic search with a manual search of reference lists. We selected RCTs comparing the effect of lifestyle intervention (exercise-plus-diet or exercise-plus-diet-plus-weight loss) versus control (no lifestyle intervention) on incident T2D defined using study-specific criteria based on a 2 h oral glucose tolerance test (OGTT) in all populations in an outpatient setting with a minimum follow-up of 1 year. We included studies that were conducted in a high-risk population including those with IGT and metabolic syndrome. Studies that only assessed exercise intervention without diet or weight-loss, used a drug(s) as part of the lifestyle intervention, or only reported observational cohort studies were excluded. In case of the multiple publication of the same trial, we used the one with the end-trial data.
3.2. Data Extraction
Two investigators (EV and TAK) independently reviewed and extracted relevant data from each included report. A standardized form was used to extract data on sample size, participant characteristics, study setting and design, level of monitoring of eating habits, intervention and control arm, macronutrient composition of diets, energy balance, follow-up duration, funding source and outcome data. All discrepancies and disagreements were resolved through consensus.
3.3. Risk of Bias Assessment
Included trials were independently assessed by two investigators (EV and TAK) for the risk of bias using the Cochrane Risk of Bias Tool [ 8 ]. An assessment was performed across 5 domains of bias (sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting). The risk of bias was assessed as either low (proper methods taken to reduce bias), high (improper methods creating bias) or unclear (insufficient information provided to determine the bias level). All discrepancies and disagreements were resolved through consensus or, where necessary, by a third author (JLS). The methods applied are described in the individual publications [ 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 ].
3.4. Data Syntheses
All analyses were conducted using Stata 16 ((StataCorp, College Station, TX, USA). Data were expressed as risk ratios (RRs) with 95% confidence intervals (CIs) and pooled using the restricted maximum likelihood (REML) random-effects models [ 29 ]. A random-effects model assumes that study estimates are estimating different, yet related, intervention effects and thus incorporates heterogeneity among studies. This is a more appropriate method to pool studies that may differ slightly in distribution of risk factors, population, size, and outcomes [ 30 ]. Heterogeneity was assessed using the Cochran Q statistic and quantified using the I 2 statistic. Significance for heterogeneity was set at p < 0.10, with an I 2 > 50% considered to be evidence of substantial heterogeneity [ 15 ]. Sources of heterogeneity were explored using sensitivity and subgroup analyses. Sensitivity analyses were performed in which each individual trial was removed from the meta-analysis and the effect size recalculated to determine whether a single trial exerted an undue influence. If ≥10 trials were available, then a priori subgroup analyses were conducted using meta-regression by baseline values, study design, follow-up, comparator arm, risk of bias and diabetes duration [ 16 ]. If ≥10 trials were available, then we also assessed publication bias by visual inspection of funnel plots and formal testing by the Egger and Begg tests [ 17 ].
3.5. Grading of the Evidence
The GRADE approach was used to assess the certainty of the evidence [ 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 ]. The certainty of the evidence was graded as high, moderate, low, or very low. Randomized controlled trials receive an initial grade of high by default and are downgraded based on the following pre-specified criteria: risk of bias (weight of trials showing risk of bias by the Cochrane Risk of Bias Tool), inconsistency (substantial unexplained inter-study heterogeneity, I 2 > 50% and p < 0.10), indirectness (presence of factors that limit the generalizability of the results), imprecision (the 95% CI for effect estimates were wide or cross minimally important differences (MIDs) for benefit or harm), and publication bias (significant evidence of small-study effects). The MID for T2D was set at 5 percent based on increased cardiovascular disease risk [ 31 ].
4.1. Search Results
Figure 1 outlines our systematic search. We identified 5286 articles from PubMed (MEDLINE), EMBASE, and Cochrane Library.
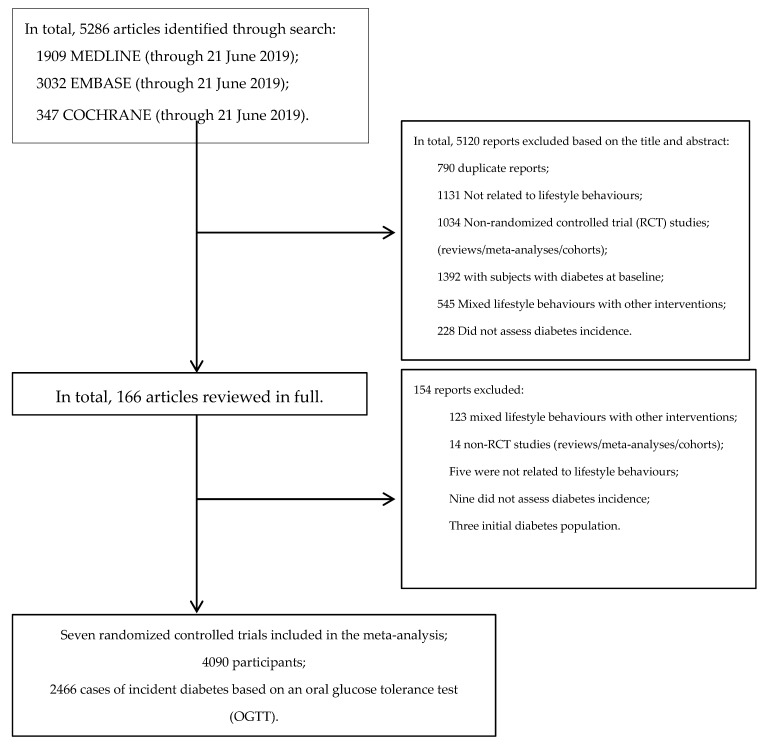
Flow diagram outlining the systematic search and article selection process.
4.2. Randomized Controlled Trials
We identified seven RCTs comprising 4090 study participants and 2466 incident type 2 diabetes cases [ 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 ] (see Table 1 and Figure 1 ). Except for the study by Bo et al. [ 38 , 39 ] (which was conducted in people with dysmetabolism), all studies were carried out in people with impaired glucose tolerance (IGT) based on an OGTT, and the diagnosis of incident diabetes was confirmed by OGTT applying contemporary WHO criteria for diabetes mellitus. Detailed data on the intervention measures and the follow-up of the control groups have been reported in individual publications and summarized in Table 1 .
Summary results on the randomized controlled trials aimed to prevent type 2 diabetes in people with impaired glucose tolerance or in people at high increased risk for diabetes.
IGT = impaired glucose tolerance based on OGTT, CHO = carbohydrates, prot = protein, SFA = saturated fatty acids, PUFA = polyunsaturated fatty acids, intervention = intervention group, control = control group, minus = reduction from baseline, NA = not available, and NS = not significant, LSM = lifestyle modification, Met = Metformin. Da Qing IGT: The Da Qing IGT and Diabetes Study; FDPS: Finnish Diabetes Prevention Study; DPP: The Diabetes Prevention Program; IDDP-1: The Indian Diabetes Prevention Programme; EDIPS: European Diabetes Prevention Study; LSM: lifestyle modification; Met: metformin; yrs: years; IGT: Impaired glucose tolerance.
4.3. Risk of Bias
Figure 2 shows the individual Cochrane Risk of Bias assessments of seven trials included in the current meta-analysis (see Figure 1 and Table 1 for details). The majority of trials were judged as having unclear or low risk of bias across domains. No evidence of a serious risk of bias was detected.
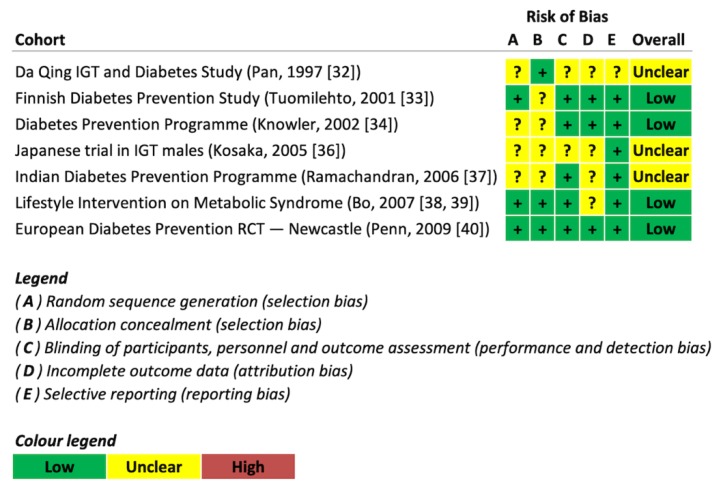
Risk of bias assessment.
4.4. Effect of Lifestyle Changes on Type 2 Diabetes Risk
Figure 3 shows the effect of lifestyle changes on T2D risk based on the meta-analysis. In seven trials involving 4090 participants [ 32 , 33 , 34 , 36 , 37 , 38 , 40 ], lifestyle intervention significantly decreased T2D risk compared to control groups (RR = 0.53 (95% CI: 0.41, 0.67), p < 0.001), with evidence of substantial inter-study heterogeneity (I 2 = 63%, p = 0.01).
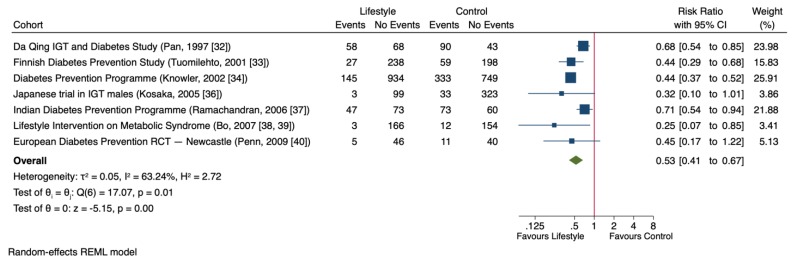
Forest plot of randomized controlled trials investigating the effect of lifestyle changes on type 2 diabetes risk (T2D). The pooled effect estimate for the overall effect is represented by the green diamond. Data are expressed as weighted risk ratios with 95% confidence intervals (CIs) using the restricted maximum likelihood (REML) random-effects model. Inter-study heterogeneity was tested by the Cochrane Q-statistic at a significance level of p < 0.10 and quantified by I 2 , where a level of ≥50% represented substantial heterogeneity.
4.5. Sensitivity and Subgroup Analyses
Table 2 shows selected sensitivity analyses in which the systematic removal of individual trials altered the results. The evidence of substantial heterogeneity was partially explained by the removal of Knowler et al. [ 34 ], which changed the evidence for heterogeneity from significant (I 2 = 65%, p = 0.009) to non-significant (I 2 = 43%, p = 0.16). However, this did not appreciably change the overall effect estimate (RR = 0.49 (95% CI: 0.37, 0.64), p < 0.001). Subgroup analyses were not conducted for any outcome as <10 trials were available.
Influence analysis assessment for the effect of lifestyle changes on T2D risk.
CI = confidence interval.
4.6. Publication Bias
Publication bias was not assessed for any outcome as <10 trials were available.
4.7. GRADE Assessment
Table 3 shows a summary of the GRADE assessments of the overall certainty of the effect of lifestyle changes on the risk of transition from IGT to T2D. The evidence was graded as high for the effect of lifestyle intervention on T2D risk reduction without any downgrading for risk of bias, inconsistency, indirectness, imprecision, or other considerations.
GRADE assessment for the effect of lifestyle changes on T2D risk.
CI = confidence interval; GRADE = grading of recommendations assessment, development, and evaluation; RR = risk ratio; T2D = type 2 diabetes. a Although there was significant heterogeneity (I 2 = 65%, p = 0.01), the removal of one study [ 34 ] explained some of the heterogeneity, which changed it from significant to non-significant (I 2 = 36%, p = 0.16). However, the estimate of effect did not change appreciably. Furthermore, this inconsistency was not considered serious as the magnitude of effect remained large and in the same direction across all the studies (RR < 0.72).
5. Discussion on the Systematic Review and Meta-Analysis
We conducted a systematic review and meta-analysis of seven randomized controlled trials involving 4090 predominantly middle-aged participants with glucose impairment (IGT or dysmetabolism), which showed that lifestyle modification including improved diet and physical activity reduced the risk of type 2 diabetes by 47 percent.
5.1. Results in the Context of Existing Literature
Recent systematic reviews published on the prevention of T2D in high-risk groups uniformly conclude that the onset of T2D can be delayed or prevented with lifestyle changes. Furthermore, these systematic reviews conclude that lifestyle changes may result in the sustained reduction of T2D [ 9 , 10 , 11 , 12 ]. On the other hand, a recent Cochrane review concluded that the evidence took into account only the combined effect of physical activity and dietary changes, and the evidence on the effect of diet or physical activity alone is insufficient [ 12 ].
A brief discussion of the included studies and other literature is helpful here as these will also be referred to in the subsequent sections of this paper. The Chinese Da Qing study [ 32 ] had altogether 577 IGT individuals in 33 study clinics that were randomized to control, exercise, healthy diet, and healthy diet plus exercise clinics, with a follow-up of 6 years. The risk of diabetes was reduced by 33% in the diet-only group, 47% in the exercise-only group and 38% in the diet-plus-exercise group as compared to the control group, without significant differences between the intervention groups. The study individuals were normal weight or overweight at baseline, and the reduction in total energy intake was 100–240 kcal depending on the intervention ( Table 1 ).
In the Finnish Diabetes Prevention Study (FDPS) [ 33 ], 522 individuals with IGT were randomized into a control or lifestyle intervention group (healthy diet and physical activity promotion). The diagnosis of T2D was based on repeated OGTT. After 3.2 years of follow-up, there was a significant decrease in the incidence of T2D, and the trial was prematurely stopped based on the decision of the independent advisory committee. The risk reduction was 58% in the intervention group compared to the control group. Weight loss was larger in the intervention group: the difference in weight reduction between the groups was 3.5 and 2.6 kg at 1 and 3 years, respectively. The intervention group also showed an increase in physical activity and the number of sedentary people was smaller in the intervention (17%) than in the control group (29%).
In the Diabetes Prevention Program (DPP) study conducted in the USA [ 34 ], altogether, 3234 individuals with IGT in 27 centers were randomized into the lifestyle intervention, metformin or control groups. The mean follow-up was 2.8 years. The risk of T2D was reduced by 58% in the lifestyle intervention group as compared to the control group. In the metformin group, the risk of diabetes was 31% lower than in the control group. At year 1, weight reduction in the intervention group was 5.6 kg and 0.1 kg in the control group. No detailed changes in physical activity were reported. It is of note that the initial BMI in the DPP was 34 kg/m 2 when in the FDPS it was 30–31 kg/m 2 .
In a Japanese study on 458 men with IGT [ 36 ], compared to the control group, a remarkable relative risk reduction of 67.4% was found in the intervention group that aimed for weight reduction, increased vegetable intake and physical activity during the 4 year follow-up. The BMI goal was 22 kg/m 2 and the majority of participants had either normal BMI or they were overweight with IGT. Still, the average weight loss was 2.2 kg in the intervention group.
In the Indian Diabetes Prevention Programme (IDPP-1) study [ 37 ], consisting of 531 subjects with IGT, there was a 28.5% reduction in the risk of T2D after 3 years of follow-up in the lifestyle modification group (LSM) compared to the control group, 28.2% reduction in the LSM-plus-metformin (Met) group and 26.4% reduction in the Met group. No significant group differences were found in the preventative effect with regard to LSM, Met and LSM-plus-Met groups. This study did not report significant changes in body weight.
Bo et al. in Italy carried out a lifestyle intervention aimed at the prevention of metabolic syndrome (MetS) in 335 subjects with dysmetabolism. This group included subjects with metabolic syndrome together with those having only two components of metabolic syndrome plus high hs-CRP values. In addition to an effect on metabolic syndrome, this study also reported 1 and 4 year results on the incidence of T2D [ 38 , 39 ]. After one year, there was a marked risk reduction in the incidence of T2D [OR 0.23; 95% CI 0.06–0.85]. The difference in weight reduction between the intervention and control groups was approximately 2.3 kg. After 4 years, the incidence of T2D was 5.4% in the intervention group and 10.2% in the control group.
In the Newcastle arm of the European Diabetes Prevention Study (EDIPS) study [ 40 ] consisting of 102 subjects with IGT, after 3 years of lifestyle intervention following mostly principles of the FDPS, the incidence of T2D was 5.0% and 11.1% in the intervention and the control groups, respectively. The average weight loss was 2.5 kg in the intervention group and sustained beneficial changes in lifestyles predicted better outcome in the T2D risk.
Before the above randomized trials that are included in the meta-analysis, Eriksson and Lindgarde reported in 1991 [ 41 ] that a 6 month sequential intervention of dietary change or increased physical activity may have prevented the development of T2D in 181 Swedish men who volunteered to take part in the lifestyle intervention compared to those who did not volunteer to participate.
In a smaller study of 88 subjects (the SLIM Study) [ 35 ], with 2 years of lifestyle intervention, not included in the current meta-analysis because it did not fulfill the inclusion criteria, there was a significant improvement in 2 h glucose values in the active intervention group. The beneficial changes could be ascribed to moderate weight loss and dietary changes (i.e., reduction in saturated fat intake) in combination with increased physical activity. Incidence data on T2D after 3 years were included in the European Diabetes Prevention Study RCT [ 42 ], where the preventative effect of ≥5% weight loss was particularly high, especially if maintained for 3 years.
Two post-hoc reports from the PREDIMED study also suggest that it is possible to prevent T2D even without significant weight loss in individuals at high risk for cardiovascular disease (CVD), using the Mediterranean diet including extra virgin olive oil or nuts. The risk reduction using the Mediterranean diet intervention, either supplemented with virgin olive oil or nuts, compared to the control group was 30% to 50% depending on the baseline population [ 43 , 44 ]. These studies are discussed in greater detail later in the manuscript with regard to the optimal diet for the prevention of T2D and cardiovascular disease.
5.2. Strengths and Limitations
Our systematic review and meta-analysis have several strengths. These include a rigorous search and selection strategy that identified all available randomized controlled trials examining the effect of lifestyle modification on T2D in individuals; the inclusion of predominantly high-quality randomized controlled trials, which give the greatest protection against bias; the use of the REML random-effects model, which is robust to non-normal distributions and has been recommended for use in meta-analyses over other random-effects estimators [ 29 ]; and the assessment of the overall certainty of the evidence using the GRADE approach.
There were no major limitations of our systematic review and meta-analysis. There was an issue of high heterogeneity, but we did not downgrade for the observed inconsistency. We did not consider the statistical heterogeneity to be a limitation as our meta-analysis included large studies with narrow confidence intervals and similar estimates in the same direction. Therefore, this apparent inconsistency was an artefact of non-overlapping narrow CIs rather than a limitation of the certainty of the overall estimate [ 23 , 45 ]. Balancing the strengths and limitations, the evidence as assessed using GRADE was of high certainty for the effect of lifestyle modification on the reduction of T2D.
6. Long-Term Results on the Prevention of Type 2 Diabetes
Three follow-up studies, the Da Qing Chinese study [ 46 ], FDPS [ 47 , 48 ] and DPP [ 49 ], showed that the beneficial lifestyle changes achieved in the prevention of T2D trials resulted in a sustained risk reduction of T2D over 10 years of follow-up ( Table 4 ).
Long-term post-intervention preventative effect on the incidence of type 2 diabetes in the former intervention groups compared to control groups in three randomized controlled lifestyle intervention studies.
Figure 4 shows the effect of lifestyle changes on the T2D risk based on the meta-analysis of the selected trials that had the long-term follow-up after the lifestyle intervention phase. In three trials consisting a total of 3855 participants with a median follow-up of 13 years [ 46 , 47 , 49 ], lifestyle intervention was associated with significantly lower T2D risk compared to control groups (RR = 0.63 [95% CI: 0.54, 0.74], p < 0.001) with no evidence of inter-study heterogeneity (I 2 = 0%, p = 0.76).
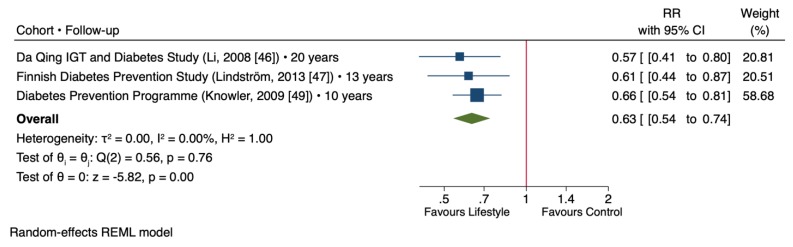
Forest plot of randomized controlled trials investigating the long-term post-intervention effect of lifestyle changes on type 2 diabetes risk. The pooled effect estimate for the overall effect is represented by the green diamond. Data are expressed as weighted risk ratios with 95% confidence intervals (CIs) using the REML random-effects model. Inter-study heterogeneity was tested by the Cochrane Q-statistic at a significance level of p < 0.10 and quantified by I 2 , where a level of ≥50% represented substantial heterogeneity.
Based on the results from FDPS [ 47 , 48 ], 22 subjects with IGT must be treated for one year or 5 subjects for five years to prevent one case of diabetes. Accordingly, in DPP [ 49 ], the respective figure was 6.9 subjects for a 3 year intervention.
7. Evidence That the Prevention of T2D in High-Risk Individuals Results in a Lower Risk of Cardiovascular Disease (CVD) and Microvascular Complications
Among the selected intervention trials, three follow-up post-intervention studies reported cardiovascular and/or microvascular complications ( Table 5 ). Furthermore, we considered the PREDIMED intervention trial results for this question as this study was carried out in high-risk individuals [ 43 , 44 ].
Long-term post-intervention data on mortality, cardiovascular (CVD) mortality and microvascular complications in the former intervention groups compared to the control groups in three randomized controlled lifestyle intervention studies.
NA: Not available.
This question is of particular importance, since the ultimate goal of the prevention and treatment of diabetes is the prevention of the long-term complications of diabetes associated with long-term hyperglycemia, dyslipidemias, hypertension, and other metabolic abnormalities, including low-grade inflammation [ 50 ]. Indeed, long-term intervention trials on the prevention of T2D have shown that besides improved glycemia, due to the correction of insulin resistance and possibly the preservation of beta-cell capacity [ 33 , 34 , 51 ], many of the well-known cardiovascular risk factors and characteristics of metabolic syndrome are corrected by changing to a healthier diet, increasing physical activity and losing weight [ 43 , 44 , 51 , 52 , 53 ]. However, there has been little evidence that the incidence of CVD or microvascular complications can be postponed or prevented by changing lifestyles. Recent data from the Da Qing Diabetes Prevention Outcome study reported results for both mortality and morbidity that suggest long-term benefits as a result of changing lifestyle habits. To summarize, there was a significant reduction in all cause deaths (26%), CVD deaths (33%) and total CVD events (26%) in the combined intervention groups as compared to the control group. Furthermore, composite microvascular diseases (35%) and the incidence of any retinopathy (40%) were significantly lower in the combined intervention groups in this cohort [ 54 ].
Furthermore, the PREDIMED study reported a significant reduction in combined stroke and all cardiovascular events in individuals randomized to the Mediterranean diet (MedDiet) plus extra-virgin olive oil or MedDiet plus nuts group [ 58 ]. Recently, the incidence of retinopathy was reported to be lower in the PREDIMED study in individuals randomized to MedDiet plus extra-virgin olive oil group (RR 0.56; 95%CI 0.32–0.97) or MedDiet plus nuts group (0.63; 95% CI 0.35–1.11). By contrast, no effect of the Mediterranean diet interventions on diabetic nephropathy was reported in the PREDIMED [ 59 ]. In the DPP follow-up study [ 55 ], retinopathic changes in women were lower in the former lifestyle intervention group than in the control group. Similarly, individuals who developed T2D had higher incidence of retinopathy than those who were non-diabetic after a long follow-up period ( Table 5 ). In FDPS, no difference was found in CVD morbidity or mortality between the intervention and control groups after 10 years, but incident cases remained low in both intervention and control groups [ 56 ]. In a sub-group analysis, the occurrence of retinopathy (microaneurysms) was significantly higher in the control (37/98, 38%) than in the intervention group (27/113, 24%; p = 0.026, see Table 4 for adjusted results) of the former FDPS participants [ 56 ].
An original report from the Look AHEAD trial showed no benefit of lifestyle intervention for the prevention of cardiovascular disease in patients with T2D, but a post-hoc analysis showed a 21% risk reduction in combined cardiovascular events in individuals who were able lose at least 10 kg of body weight as compared to patients with a stable body weight or long-term weight gain [ 60 ].
A recent systematic review and meta-analysis of prospective cohort studies and randomized clinical trials suggests that MedDiet has a beneficial role on the CVD prevention in populations inclusive of the individuals with T2D [ 61 ].
Discussion on Macro- and Microvascular Risk Reduction in the T2D Prevention Trials
Among the diabetes prevention trials which have examined follow-up data, only the Chinese Da Qing Diabetes Prevention Outcome Study has reported lower mortality and morbidity from any cause and cardiovascular disease in the people with IGT randomized into lifestyle intervention groups ( Table 5 ). Furthermore, the Chinese study found a clear decrease in composite microvascular diseases and retinopathy [ 54 ]. Indeed, these long-term results are of particular interest, since one long-term goal of the prevention of T2D is to prevent its complications as well. A longer follow-up of a relatively younger age cohort that is also less obese is a possible reason why significant risk reduction in CVD mortality and morbidity is only seen in the Chinese study and not in the American DPP Outcome Study [ 55 ] or in the FDPS [ 56 ]. After the active intervention phase, both the American and Finnish study participants, on average, remained relatively obese compared to the Chinese study. There may also be genetic or ethnic differences between the study populations, resulting in different distributions of the risk factors for T2D and of T2D rate itself [ 3 ]. For example, smoking was particularly common among the Chinese study participants [ 54 ]. Furthermore, the management of the main risk factors and health care resources available may offer other explanations for divergent results. In terms of microvascular complications, which are closely associated to hyperglycemia, the Chinese study results were encouraging with a 35% reduction in composite microvascular complications and 40% reduction in any retinopathy in the intervention groups. The results from both the DPP Outcome Study and the FDPS supported the long-term benefit achieved by changing lifestyles with regard to incident retinopathy [ 55 , 57 ]. Finally, it should be emphasized that the statistical power of the intervention studies on the prevention of T2D may not be sufficient to show significant differences in CVD outcomes between the intervention and the control groups [ 62 ].
8. Discussion on the Factors Explaining the Risk Reduction of T2D Including the Optimal Dietary Composition for the Prevention of T2D
8.1. what are the factors explaining the risk reduction of t2d in randomized controlled trials.
This question is of particular importance as it is related to strategies in preventing T2D. The Da Qing IGT study is the only study with both diet and physical activity arms randomized by clinic [ 32 ], and the PREDIMED trial is the only study testing the effect of a food pattern enriched with key foods (nuts or virgin olive oil) without physical activity or energy restriction [ 43 , 44 ]. All other lifestyle intervention studies combine dietary changes, weight reduction for overweight or obese people, and physical activity. It is of note that Chinese people with IGT in the Da Qing study [ 32 ], Japanese men with IGT [ 36 ], and individuals in the Indian IDD-1 study [ 37 ] had a much lower BMI than in study populations carried out in Europe or in the U.S.A.
8.2. Weight Reduction
Based on secondary analyses of randomized controlled trials, it can be concluded that a better adherence to lifestyle changes in general results in the better long-term prevention of T2D [ 33 , 48 , 49 ]. Furthermore, based on the evidence coming from observational studies on T2D risk factors [ 2 , 63 ] and the remarkable beneficial effects of weight reduction on glucose metabolism [ 51 , 64 , 65 , 66 ], weight reduction has been considered as a cornerstone in the prevention of T2D; with larger weight reductions associated with a lower risk of T2D. In the EDIPS study on 771 participants with IGT combining data from the FDPS, and SLIM and Newcastle studies, the risk of T2D was 89% lower in individuals who were able to sustain weight loss of at least 5% over 3 years than in individuals without significant weight changes [ 42 ]. Nevertheless, it is impossible to conclude that weight reduction is the only means to reduce the risk of T2D in overweight and obese people with impaired glucose metabolism, since weight loss is almost always associated with simultaneous changes in physical activity and/or diet. Indeed, the studies in people with Asian origin suggest that changing diet and increasing physical activity also seem to play a significant role in the prevention of T2D in individuals at risk for T2D with both normal body weight and over-weight people [ 32 , 36 , 37 ]. The importance of weight reduction in T2D can be gauged from a recent weight-management trial, in which 306 individuals with T2D in 39 primary care practices demonstrated a remission rate of 86% in individuals who lost 15 kg or more (24% of participants) [ 67 ]; an overall weight-loss difference of 9 kg resulted in a remission rate of 46% in the intervention group versus 4% in the control group in the full study.
8.3. Optimal Diet
8.3.1. individual nutrients and foods.
Several observational studies have been conducted to analyze the associations between food groups or nutrient consumption and T2D incidence. Ley et al. [ 68 ] conducted a series of meta-analyses of prospective cohort studies on food and beverage intake and T2D risk. Processed and unprocessed red meat, white rice, and sugar-sweetened beverages have shown a consistent positive relation with T2D, whereas green leafy vegetables, total dairy products, whole grains, alcohol in moderation in women, and coffee have been inversely associated with T2D. The consumption of berries and fruits rich in anthocyanins, such as bilberries, blueberries, grapes, apples, and pears, has also been associated with a lower risk of T2D [ 69 ]. Recent evidence also shows that yogurt intake [ 70 ] and nut intake (in women) is inversely associated with T2D. Legumes are another food group with cardiometabolic benefits [ 71 , 72 , 73 , 74 , 75 , 76 , 77 ] and legumes show an inverse association with the risk of diabetes and gestational diabetes [ 77 , 78 ]. In the same meta-analysis of prospective studies by Ley et al. [ 68 ], heme-iron, glycemic index and glycemic load of the diet were directly associated with T2D incidence, whereas total magnesium and vitamin D in the diet, as well as cereal fiber, were inversely related to T2D. A recent review based on meta-analyses and earlier reviews emphasize the preventive effect of whole grains and dietary fiber on the incidence of T2D [ 79 ].
8.3.2. Dietary Patterns
In addition to individual nutrients and foods, several studies have looked at dietary patterns and prevention of T2D. A Western dietary pattern, which is high in sugar-sweetened soft drinks, refined grains, diet soft drinks, and processed meat, was associated with an increased risk of diabetes in the Nurses Health Study (NHS) I and NHS II studies [ 80 ].
In contrast, some prospective cohort studies have demonstrated that adherence to plant-based dietary patterns, such as Mediterranean [ 81 , 82 ] DASH (Dietary Approaches to Stop Hypertension) or vegetarian dietary patterns [ 82 , 83 , 84 , 85 ], are associated with a lower risk of T2D incidence. In two prospective studies, a Mediterranean-type or healthy dietary pattern has also been inversely related to gestational diabetes [ 78 , 86 ].
Meal frequency and timing may also have a role in the T2D risk. Skipping breakfast and snacking have been associated with increased risk of T2D in both men and women [ 87 , 88 ]. Based on limited evidence, consuming breakfast regularly and not eating snacks between main meals may also be a strategy to reduce the risk of T2D [ 89 ].
8.3.3. Diet and Weight Loss
Current evidence from randomized intervention trials ( Table 1 ) suggests that weight loss by means of a healthy diet with lower saturated fat intake, but rich in vegetables, fruit, and whole grain products is beneficial in the prevention of T2D, especially when combined with physical activity. Indeed, all of the seven randomized lifestyle intervention studies in our systematic review and meta-analysis applied this kind of dietary approach. In FDPS, the best results in the prevention of T2D were achieved in IGT individuals with high fiber but moderate fat intake [ 47 , 90 ]. Similarly, in the American DPP study, 1 year weight loss success was associated with a high carbohydrate, high fiber, but a rather low total and saturated fat diet intake [ 91 ]. Regarding the quality of dietary fat, current evidence suggests that unsaturated fatty acids may have beneficial effects on insulin sensitivity and it is suggested to lower the risk of T2D [ 92 , 93 ].
In the PREDIMED trial, the Mediterranean diet enriched in nuts or extra virgin olive oil, resulted in a significant reduction in the incidence of T2D independent of weight loss or physical activity changes. This suggests that the quality of the diet may play a role in the prevention of T2D independent of weight changes [ 43 , 44 ]. However, these results are based on post-hoc analyses of a population at high cardiovascular risk and may not be extrapolated to healthy populations. In the SLIM and Newcastle studies, better adherence to the diet also predicted lower T2D risk [ 42 ]. To conclude, a diet with low consumption of red and processed meat, sugar, and sugar-sweetened beverages, but rich in vegetables, fruit, legumes, and whole grain products seems to be beneficial in the prevention of T2D.
8.3.4. Physical Activity
The Chinese Da Qing study [ 32 ] is the only intervention study that has examined the effect of exercise without weight loss or dietary changes. In the physical activity clinics, the risk of T2D was reduced by 47% as compared to clinics serving as control clinics, but no significant differences were observed between different randomization groups ( Table 1 ). There are no other long-term controlled intervention trials in this field. In FDPS, the impact of physical activity was examined as a secondary analysis taking into account the effect of diet and weight reduction. Based on different criteria used to evaluate physical activity, it was concluded that being physically active may reduce T2D risk by approximately 50% [ 94 ]. The recommendations to increase physical activity are strongly grounded by short-term controlled interventions that show improved glucose metabolism after increasing physical activity. Furthermore, epidemiological and trial evidence support the view that physical inactivity/sedentary lifestyle, along with being overweight and/or obese, are important risk factors for T2D and contribute to the current epidemic of T2D [ 1 , 2 , 95 , 96 ]. A recent PREDIMED-Plus Trial on overweight/obese individuals with metabolic syndrome who combined an energy-reduced Mediterranean-type diet and exercise promotion showed significant weight reduction (3.2 vs. 0.7 kg) and improvements in glucose metabolism, serum concentrations of triglycerides, HDL-cholesterol, and some inflammatory factors, compared to controls. These results confirm that a multifactorial approach, including physical activity, is successful in the prevention and treatment of disturbances in glucose metabolism [ 52 ].
9. Conclusions
We have a high certainty of evidence that T2D is preventable by changing lifestyle, i.e., weight reduction by diet change according to the current recommendations in terms of quality of fat, fiber intake, increased use of whole grain products, fruit, and vegetables, and increasing physical activity. The risk reduction of T2D is strongly related to the degree of long-term weight loss and adherence to lifestyle changes, and this preventive effect has been demonstrated to sustain for many years after active intervention.
Additional well-controlled intervention studies are needed to identify the optimal diet to prevent T2D. Currently, a diet moderate in fat, low in saturated fat intake, rich in fiber, whole grains, and fruit and vegetables, as well as a Mediterranean-type diet, may be recommended for the prevention of T2D in prediabetes.
There is still limited/insufficient evidence that the prevention of T2D by changing lifestyle may also prevent CVD or microvascular diseases.
Author Contributions
Conceptualization, M.U., H.K., A.A.R., K.H., A.P., A.T., J.S.-S., U.S. and J.L.S.; Methodology, M.U., T.A.K., E.V. and J.L.S.; Software, T.A.K. and E.V.; Validation, M.U., E.V. and T.A.K.; Formal Analysis, E.V., T.A.K.; Investigation, M.U., E.V., T.A.K. and J.L.S.; Resources, M.U., E.V., T.A.K., and J.L.S.; Data Curation, M.U., E.V. and T.A.K.; Writing—Original Draft Preparation, M.U., E.V., T.A.K., and J.L.S.; Writing—Review & Editing, M.U., T.A.K., E.V., H.K., A.A.R., K.H., A.P., A.T., J.S.-S., U.S. and J.L.S.; Visualization, M.U. and T.A.K.; Supervision, M.U. and J.L.S.; Project Administration, M.U. and J.L.S.; Funding Acquisition, M.U. and J.L.S.
The Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD) commissioned this systematic review and meta-analysis and provided funding and logistical support for meetings as part of the development of the EASD Clinical Practice Guidelines for Nutrition Therapy. This work was also supported by the Canadian Institutes of Health Research [funding reference number, 129920] through the Canada-wide Human Nutrition Trialists’ Network (NTN). The Diet, Digestive tract, and Disease (3-D) Centre, funded through the Canada Foundation for Innovation (CFI) and the Ministry of Research and Innovation’s Ontario Research Fund (ORF), provided the infrastructure for the conduct of this project. Effie Viguiliouk was supported by a Toronto 3D Knowledge Synthesis and Clinical Trials foundation Internship Award. John L Sievenpiper was funded by a Diabetes Canada Clinician Scientist award. With the exception of the Clinical Practice Guidelines Committee of the DNSG of the EASD, none of the sponsors had a role in any aspect of the present study, including the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, approval of the manuscript or decision to publish.
Conflicts of Interest
JSS serves on the board of, and has received a grant through, his institution from the International Nut and Dried Fruit Council and the Eroski Foundation. He serves on the Executive Committee of the Instituto Danone Spain and on the Scientific Committee of the Danone International Institute. He has received research support from the Instituto de Salud Carlos III, Spain; the Ministerio de Educación y Ciencia, Spain; the Departament de Salut Pública de la Generalitat de Catalunya, Catalonia, Spain; and the European Commission. Further research support has come from the California Walnut Commission, Sacramento CA, USA; the Patrimonio Comunal Olivarero, Spain; the La Morella Nuts, Spain; and Borges S.A., Spain. He reports receiving consulting fees or travel expenses from Danone; California Walnut Commission, the Eroski Foundation, the Instituto Danone–Spain, Nuts for Life, Australian Nut Industry Council, Nestlé, Abbot Laboratories, and Font Vella Lanjarón. He is on the Clinical Practice Guidelines Expert Committee of the European Association for the study of Diabetes (EASD) and has served on the Scientific Committee of the Spanish Food and Safety Agency, and the Spanish Federation of the Scientific Societies of Food, Nutrition and Dietetics. He is an Executive Board Member of the Diabetes and Nutrition Study Group [DNSG] of the EASD. JLS has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, Canadian Institutes of health Research (CIHR), Diabetes Canada, PSI Foundation, Banting and Best Diabetes Centre (BBDC), American Society for Nutrition (ASN), INC International Nut and Dried Fruit Council Foundation, National Dried Fruit Trade Association, The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), and the Nutrition Trialists Fund at the University of Toronto (a fund established by an inaugural donation from the Calorie Control Council). He has received in-kind food donations to support a randomized controlled trial from the Almond Board of California, California Walnut Commission, American Peanut Council, Barilla, Unilever, Unico/Primo, Loblaw Companies, Quaker, Kellogg Canada, and WhiteWave Foods. He has received travel support, speaker fees and/or honoraria from Diabetes Canada, Mott’s LLP, Dairy Farmers of Canada, FoodMinds LLC, International Sweeteners Association, Nestlé, Pulse Canada, Canadian Society for Endocrinology and Metabolism (CSEM), GI Foundation, Abbott, Biofortis, ASN, Northern Ontario School of Medicine, INC Nutrition Research & Education Foundation, European Food Safety Authority (EFSA), Comité Européen des Fabricants de Sucre (CEFS), and Physicians Committee for Responsible Medicine. He has or has had ad hoc consulting arrangements with Perkins Coie LLP, Tate & Lyle, and Wirtschaftliche Vereinigung Zucker e.V. He is a member of the European Fruit Juice Association Scientific Expert Panel. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Obesity Canada. He serves or has served as an unpaid scientific advisor for the Food, Nutrition, and Safety Program (FNSP) and the Technical Committee on Carbohydrates of the International Life Science Institute (ILSI) North America. He is a member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His wife is an employee of Sobeys Inc. TAK has received research support from the Canadian Institutes of Health Research (CIHR) and an unrestricted travel donation from Bee Maid Honey Ltd. He was an invited speaker at a Calorie Control Council annual general meeting for which he received an honorarium. No competing interests were declared by the other authors (MU, EV, HK, AAR, KH, AP, AT, US).
- 1. International Diabetes Federation . IDF Atlas. 8th ed. International Diabetes Federation; Brussels, Belgium: 2017. [ Google Scholar ]
- 2. World Health Organization . Global Report on Diabetes 2016. WHO; Geneva, Switzerland: 2017. [ Google Scholar ]
- 3. Davies M.J., D’Alessio D.A., Fradkin J., Kernan W.N., Mathieu C., Mingrone G., Rossing P., Tsapas A., Wexler D.J., Buse J.B. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2018;61:2461–2498. doi: 10.1007/s00125-018-4729-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Gloy V.L., Briel M., Bhatt D.L., Kashyap S.R., Schauer P.R., Mingrone G., Bucher H.C., Nordmann A.J. Bariatric surgery versus non-surgical treatment for obesity: A systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:5934. doi: 10.1136/bmj.f5934. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Douglas I.J., Bhaskaran K., Batterham R.L., Smeeth L. Bariatric Surgery in the United Kingdom: A Cohort Study of Weight Loss and Clinical Outcomes in Routine Clinical Care. PLoS Med. 2015;12:e1001925. doi: 10.1371/journal.pmed.1001925. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. World Health Organization . Diabetes Mellitus, Report of a WHO Study Group. WHO; Geneva, Switzerland: 1985. WHO Technical Report. [ PubMed ] [ Google Scholar ]
- 7. Mann J.I., De Leeuw I., Hermansen K., Karamanos B., Karlstrom B., Katsilambros N., Riccardi G., Rivellese A.A., Rizkalla S., Slama G., et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2004;14:373–394. doi: 10.1016/S0939-4753(04)80028-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Higgins J.P.T., Green S. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collaboration; Oxford, UK: 2011. [ Google Scholar ]
- 9. Schellenberg E.S., Dryden D.M., Vandermeer B., Ha C., Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2013;159:543–551. doi: 10.7326/0003-4819-159-8-201310150-00007. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Barry E., Roberts S., Oke J., Vijayaraghavan S., Normansell R., Greenhalgh T. Efficacy and effectiveness of screen and treat policies in prevention of type 2 diabetes: Systematic review and meta-analysis of screening tests and interventions. BMJ. 2017;356:6538. doi: 10.1136/bmj.i6538. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Haw J.S., Galaviz K.I., Straus A.N., Kowalski A.J., Magee M.J., Weber M.B., Wei J., Narayan K.M.V., Ali M.K. Long-term Sustainability of Diabetes Prevention Approaches: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Intern. Med. 2017;177:1808–1817. doi: 10.1001/jamainternmed.2017.6040. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Hemmingsen B., Gimenez-Perez G., Mauricio D., Roque I.F.M., Metzendorf M.I., Richter B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2017;12:003054. doi: 10.1002/14651858.CD003054.pub4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Brunetti M., Shemilt I., Pregno S., Vale L., Oxman A.D., Lord J., Sisk J., Ruiz F., Hill S., Guyatt G.H., et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. J. Clin. Epidemiol. 2013;66:140–150. doi: 10.1016/j.jclinepi.2012.04.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Balshem H., Helfand M., Schunemann H.J., Oxman A.D., Kunz R., Brozek J., Vist G.E., Falck-Ytter Y., Meerpohl J., Norris S., et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Higgins J.P.T., Green S. Cochrane Handbook for Systematic Reviews of Interventions. Wiley; Hoboken, NJ, USA: 2008. p. 672. [ Google Scholar ]
- 16. Thompson S.G., Higgins J.P. How should meta-regression analyses be undertaken and interpreted? Stat. Med. 2002;21:1559–1573. doi: 10.1002/sim.1187. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Sterne J.A., Gavaghan D., Egger M. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J. Clin. Epidemiol. 2000;53:1119–1129. doi: 10.1016/S0895-4356(00)00242-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Guyatt G., Oxman A.D., Akl E.A., Kunz R., Vist G., Brozek J., Norris S., Falck-Ytter Y., Glasziou P., DeBeer H., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Guyatt G., Oxman A.D., Sultan S., Brozek J., Glasziou P., Alonso-Coello P., Atkins D., Kunz R., Montori V., Jaeschke R., et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J. Clin. Epidemiol. 2013;66:151–157. doi: 10.1016/j.jclinepi.2012.01.006. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Guyatt G.H., Oxman A.D., Kunz R., Atkins D., Brozek J., Vist G., Alderson P., Glasziou P., Falck-Ytter Y., Schunemann H.J. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J. Clin. Epidemiol. 2011;64:395–400. doi: 10.1016/j.jclinepi.2010.09.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Guyatt G.H., Oxman A.D., Kunz R., Brozek J., Alonso-Coello P., Rind D., Devereaux P.J., Montori V.M., Freyschuss B., Vist G., et al. GRADE guidelines 6. Rating the quality of evidence—Imprecision. J. Clin. Epidemiol. 2011;64:1283–1293. doi: 10.1016/j.jclinepi.2011.01.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Guyatt G.H., Oxman A.D., Kunz R., Woodcock J., Brozek J., Helfand M., Alonso-Coello P., Falck-Ytter Y., Jaeschke R., Vist G., et al. GRADE guidelines: 8. Rating the quality of evidence—Indirectness. J. Clin. Epidemiol. 2011;64:1303–1310. doi: 10.1016/j.jclinepi.2011.04.014. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Guyatt G.H., Oxman A.D., Kunz R., Woodcock J., Brozek J., Helfand M., Alonso-Coello P., Glasziou P., Jaeschke R., Akl E.A., et al. GRADE guidelines: 7. Rating the quality of evidence—Inconsistency. J. Clin. Epidemiol. 2011;64:1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Guyatt G.H., Oxman A.D., Montori V., Vist G., Kunz R., Brozek J., Alonso-Coello P., Djulbegovic B., Atkins D., Falck-Ytter Y., et al. GRADE guidelines: 5. Rating the quality of evidence—Publication bias. J. Clin. Epidemiol. 2011;64:1277–1282. doi: 10.1016/j.jclinepi.2011.01.011. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Guyatt G.H., Oxman A.D., Santesso N., Helfand M., Vist G., Kunz R., Brozek J., Norris S., Meerpohl J., Djulbegovic B., et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J. Clin. Epidemiol. 2013;66:158–172. doi: 10.1016/j.jclinepi.2012.01.012. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Guyatt G.H., Oxman A.D., Sultan S., Glasziou P., Akl E.A., Alonso-Coello P., Atkins D., Kunz R., Brozek J., Montori V., et al. GRADE guidelines: 9. Rating up the quality of evidence. J. Clin. Epidemiol. 2011;64:1311–1316. doi: 10.1016/j.jclinepi.2011.06.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Guyatt G.H., Oxman A.D., Vist G., Kunz R., Brozek J., Alonso-Coello P., Montori V., Akl E.A., Djulbegovic B., Falck-Ytter Y., et al. GRADE guidelines: 4. Rating the quality of evidence—Study limitations (risk of bias) J. Clin. Epidemiol. 2011;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Guyatt G.H., Thorlund K., Oxman A.D., Walter S.D., Patrick D., Furukawa T.A., Johnston B.C., Karanicolas P., Akl E.A., Vist G., et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J. Clin. Epidemiol. 2013;66:173–183. doi: 10.1016/j.jclinepi.2012.08.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Langan D., Higgins J.P.T., Jackson D., Bowden J., Veroniki A.A., Kontopantelis E., Viechtbauer W., Simmonds M. A comparison of heterogeneity variance estimators in simulated random-effects meta-analyses. Res. Synth. Methods. 2019;10:83–98. doi: 10.1002/jrsm.1316. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Riley R.D., Higgins J.P., Deeks J.J. Interpretation of random effects meta-analyses. BMJ. 2011;342:549. doi: 10.1136/bmj.d549. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Booth G.L., Kapral M.K., Fung K., Tu J.V. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: A population-based retrospective cohort study. Lancet. 2006;368:29–36. doi: 10.1016/S0140-6736(06)68967-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Pan X.R., Li G.W., Hu Y.H., Wang J.X., Yang W.Y., An Z.X., Hu Z.X., Lin J., Xiao J.Z., Cao H.B., et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Tuomilehto J., Lindstrom J., Eriksson J.G., Valle T.T., Hamalainen H., Ilanne-Parikka P., Keinanen-Kiukaanniemi S., Laakso M., Louheranta A., Rastas M., et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A., Nathan D.M. Diabetes Prevention Program Research, G. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Mensink M., Blaak E.E., Corpeleijn E., Saris W.H., de Bruin T.W., Feskens E.J. Lifestyle intervention according to general recommendations improves glucose tolerance. Obes. Res. 2003;11:1588–1596. doi: 10.1038/oby.2003.211. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Kosaka K., Noda M., Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: A Japanese trial in IGT males. Diabetes Res. Clin. Pract. 2005;67:152–162. doi: 10.1016/j.diabres.2004.06.010. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Ramachandran A., Snehalatha C., Mary S., Mukesh B., Bhaskar A.D., Vijay V. Indian Diabetes Prevention, P. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Bo S., Ciccone G., Baldi C., Benini L., Dusio F., Forastiere G., Lucia C., Nuti C., Durazzo M., Cassader M., et al. Effectiveness of a lifestyle intervention on metabolic syndrome. A randomized controlled trial. J. Gen. Intern. Med. 2007;22:1695–1703. doi: 10.1007/s11606-007-0399-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Bo S., Gambino R., Ciccone G., Rosato R., Milanesio N., Villois P., Pagano G., Cassader M., Gentile L., Durazzo M., et al. Effects of TCF7L2 polymorphisms on glucose values after a lifestyle intervention. Am. J. Clin. Nutr. 2009;90:1502–1508. doi: 10.3945/ajcn.2009.28379. [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Penn L., White M., Oldroyd J., Walker M., Alberti K.G., Mathers J.C. Prevention of type 2 diabetes in adults with impaired glucose tolerance: The European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC Public Health. 2009;9:342. doi: 10.1186/1471-2458-9-342. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 41. Eriksson K.F., Lindgarde F. Prevention of type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. The 6-year Malmo feasibility study. Diabetologia. 1991;34:891–898. doi: 10.1007/BF00400196. [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Penn L., White M., Lindstrom J., den Boer A.T., Blaak E., Eriksson J.G., Feskens E., Ilanne-Parikka P., Keinanen-Kiukaanniemi S.M., Walker M., et al. Importance of weight loss maintenance and risk prediction in the prevention of type 2 diabetes: Analysis of European Diabetes Prevention Study RCT. PLoS ONE. 2013;8:e57143. doi: 10.1371/journal.pone.0057143. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Salas-Salvado J., Bullo M., Babio N., Martinez-Gonzalez M.A., Ibarrola-Jurado N., Basora J., Estruch R., Covas M.I., Corella D., Aros F., et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: Results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011;34:14–19. doi: 10.2337/dc10-1288. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Salas-Salvado J., Bullo M., Estruch R., Ros E., Covas M.I., Ibarrola-Jurado N., Corella D., Aros F., Gomez-Gracia E., Ruiz-Gutierrez V., et al. Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Ann. Intern. Med. 2014;160:1–10. doi: 10.7326/M13-1725. [ DOI ] [ PubMed ] [ Google Scholar ]
- 45. Rücker G., Schwarzer G., Carpenter J.R., Schumacher M. Undue reliance on I(2) in assessing heterogeneity may mislead. BMC Med. Res. Methodol. 2008;8:79. doi: 10.1186/1471-2288-8-79. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Li G., Zhang P., Wang J., Gregg E.W., Yang W., Gong Q., Li H., Li H., Jiang Y., An Y., et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: A 20-year follow-up study. Lancet. 2008;371:1783–1789. doi: 10.1016/S0140-6736(08)60766-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Lindstrom J., Peltonen M., Eriksson J.G., Ilanne-Parikka P., Aunola S., Keinanen-Kiukaanniemi S., Uusitupa M., Tuomilehto J. Improved lifestyle and decreased diabetes risk over 13 years: Long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS) Diabetologia. 2013;56:284–293. doi: 10.1007/s00125-012-2752-5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Lindstrom J., Ilanne-Parikka P., Peltonen M., Aunola S., Eriksson J.G., Hemio K., Hamalainen H., Harkonen P., Keinanen-Kiukaanniemi S., Laakso M., et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: Follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Diabetes Prevention Program Research Group 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 50. Nathan D.M. Long-term complications of diabetes mellitus. N. Engl. J. Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [ DOI ] [ PubMed ] [ Google Scholar ]
- 51. de Mello V.D., Lindström J., Eriksson J., Ilanne-Parikka P., Keinänen-Kiukaanniemi S., Sundvall J., Laakso M., Tuomilehto J., Uusitupa M. Insulin secretion and its determinants in the progression of impaired glucose tolerance to type 2 diabetes in impaired glucose-tolerant individuals: The Finnish Diabetes Prevention Study. Diabetes Care. 2012;35:211–217. doi: 10.2337/dc11-1272. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Salas-Salvado J., Diaz-Lopez A., Ruiz-Canela M., Basora J., Fito M., Corella D., Serra-Majem L., Warnberg J., Romaguera D., Estruch R., et al. Effect of a Lifestyle Intervention Program with Energy-Restricted Mediterranean Diet and Exercise on Weight Loss and Cardiovascular Risk Factors: One-Year Results of the PREDIMED-Plus Trial. Diabetes Care. 2019;42:777–788. doi: 10.2337/dc18-0836. [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Uusitupa M., Lindstrom J., Tuomilehto J. Prevention of type 2 diabetes-success story that is waiting for next steps. Eur. J. Clin. Nutr. 2018;72:1260–1266. doi: 10.1038/s41430-018-0223-x. [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Gong Q., Gregg E.W., Wang J., An Y., Zhang P., Yang W., Li H., Li H., Jiang Y., Shuai Y., et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: The China Da Qing Diabetes Prevention Outcome Study. Diabetologia. 2011;54:300–307. doi: 10.1007/s00125-010-1948-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 55. Diabetes Prevention Program Research Group Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3:866–875. doi: 10.1016/S2213-8587(15)00291-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 56. Uusitupa M., Peltonen M., Lindstrom J., Aunola S., Ilanne-Parikka P., Keinanen-Kiukaanniemi S., Valle T.T., Eriksson J.G., Tuomilehto J. Ten-year mortality and cardiovascular morbidity in the Finnish Diabetes Prevention Study—Secondary analysis of the randomized trial. PLoS ONE. 2009;4:e5656. doi: 10.1371/journal.pone.0005656. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 57. Aro A., Kauppinen A., Kivinen N., Selander T., Kinnunen K., Tuomilehto J., Keinanen-Kiukaanniemi S., Lindstrom J., Uusitupa M., Kaarniranta K. Life Style Intervention Improves Retinopathy Status-The Finnish Diabetes Prevention Study. Nutrients. 2019;11:1691. doi: 10.3390/nu11071691. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Estruch R., Ros E., Salas-Salvado J., Covas M.I., Corella D., Aros F., Gomez-Gracia E., Ruiz-Gutierrez V., Fiol M., Lapetra J., et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389. [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Diaz-Lopez A., Babio N., Martinez-Gonzalez M.A., Corella D., Amor A.J., Fito M., Estruch R., Aros F., Gomez-Gracia E., Fiol M., et al. Mediterranean Diet, Retinopathy, Nephropathy, and Microvascular Diabetes Complications: A Post Hoc Analysis of a Randomized Trial. Diabetes Care. 2015;38:2134–2141. doi: 10.2337/dc15-1117. [ DOI ] [ PubMed ] [ Google Scholar ]
- 60. Look Ahead Research Group. Gregg E.W., Jakicic J.M., Blackburn G., Bloomquist P., Bray G.A., Clark J.M., Coday M., Curtis J.M., Egan C., et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: A post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4:913–921. doi: 10.1016/S2213-8587(16)30162-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Becerra-Tomas N., Blanco Mejia S., Viguiliouk E., Khan T., Kendall C.W.C., Kahleova H., Rahelic D., Sievenpiper J.L., Salas-Salvado J. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2019 doi: 10.1080/10408398.2019.1565281. [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Nathan D.M., Bennett P.H., Crandall J.P., Edelstein S.L., Goldberg R.B., Kahn S.E., Knowler W.C., Mather K.J., Mudaliar S., Orchard T.J., et al. Does diabetes prevention translate into reduced long-term vascular complications of diabetes? Diabetologia. 2019;62:1319–1328. doi: 10.1007/s00125-019-4928-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Aschner P. New IDF clinical practice recommendations for managing type 2 diabetes in primary care. Diabetes Res. Clin. Pract. 2017;132:169–170. doi: 10.1016/j.diabres.2017.09.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 64. Uusitupa M., Lindi V., Louheranta A., Salopuro T., Lindstrom J., Tuomilehto J. Long-term improvement in insulin sensitivity by changing lifestyles of people with impaired glucose tolerance: 4-year results from the Finnish Diabetes Prevention Study. Diabetes. 2003;52:2532–2538. doi: 10.2337/diabetes.52.10.2532. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Kitabchi A.E., Temprosa M., Knowler W.C., Kahn S.E., Fowler S.E., Haffner S.M., Andres R., Saudek C., Edelstein S.L., Arakaki R., et al. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the diabetes prevention program: effects of lifestyle intervention and metformin. Diabetes. 2005;54:2404–2414. doi: 10.2337/diabetes.54.8.2404. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Hamman R.F., Horton E., Barrett-Connor E., Bray G.A., Christophi C.A., Crandall J., Florez J.C., Fowler S., Goldberg R., Kahn S.E., et al. Factors affecting the decline in incidence of diabetes in the Diabetes Prevention Program Outcomes Study (DPPOS) Diabetes. 2015;64:989–998. doi: 10.2337/db14-0333. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Lean M.E., Leslie W.S., Barnes A.C., Brosnahan N., Thom G., McCombie L., Peters C., Zhyzhneuskaya S., Al-Mrabeh A., Hollingsworth K.G., et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): An open-label, cluster-randomised trial. Lancet. 2018;391:541–551. doi: 10.1016/S0140-6736(17)33102-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- 68. Ley S.H., Hamdy O., Mohan V., Hu F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet. 2014;383:1999–2007. doi: 10.1016/S0140-6736(14)60613-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 69. Muraki I., Imamura F., Manson J.E., Hu F.B., Willett W.C., van Dam R.M., Sun Q. Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. BMJ. 2013;347:f5001. doi: 10.1136/bmj.f5001. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Salas-Salvado J., Guasch-Ferre M., Diaz-Lopez A., Babio N. Yogurt and Diabetes: Overview of Recent Observational Studies. J. Nutr. 2017;147:1452–1461. doi: 10.3945/jn.117.248229. [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Meyer K.A., Kushi L.H., Jacobs D.R., Slavin J., Sellers T.A., Folsom A.R. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am. J. Clin. Nutr. 2000;71:921–930. doi: 10.1093/ajcn/71.4.921. [ DOI ] [ PubMed ] [ Google Scholar ]
- 72. Hodge A.M., English D.R., O’Dea K., Giles G.G. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care. 2004;27:2701–2706. doi: 10.2337/diacare.27.11.2701. [ DOI ] [ PubMed ] [ Google Scholar ]
- 73. Bazzano L.A., Li T.Y., Joshipura K.J., Hu F.B. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes care. 2008;31:1311–1317. doi: 10.2337/dc08-0080. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 74. Villegas R., Gao Y.T., Yang G., Li H.L., Elasy T.A., Zheng W., Shu X.O. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women’s Health Study. Am. J. Clin. Nutr. 2008;87:162–167. doi: 10.1093/ajcn/87.1.162. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 75. Ericson U., Sonestedt E., Gullberg B., Hellstrand S., Hindy G., Wirfalt E., Orho-Melander M. High intakes of protein and processed meat associate with increased incidence of type 2 diabetes. Br. J. Nutr. 2013;109:1143–1153. doi: 10.1017/S0007114512003017. [ DOI ] [ PubMed ] [ Google Scholar ]
- 76. von Ruesten A., Feller S., Bergmann M.M., Boeing H. Diet and risk of chronic diseases: Results from the first 8 years of follow-up in the EPIC-Potsdam study. Eur. J. Clin. Nutr. 2013;67:412–419. doi: 10.1038/ejcn.2013.7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 77. Becerra-Tomas N., Diaz-Lopez A., Rosique-Esteban N., Ros E., Buil-Cosiales P., Corella D., Estruch R., Fito M., Serra-Majem L., Aros F., et al. Legume consumption is inversely associated with type 2 diabetes incidence in adults: A prospective assessment from the PREDIMED study. Clin. Nutr. 2018;37:906–913. doi: 10.1016/j.clnu.2017.03.015. [ DOI ] [ PubMed ] [ Google Scholar ]
- 78. Karamanos B., Thanopoulou A., Anastasiou E., Assaad-Khalil S., Albache N., Bachaoui M., Slama C.B., El Ghomari H., Jotic A., Lalic N., et al. Relation of the Mediterranean diet with the incidence of gestational diabetes. Eur. J. Clin. Nutr. 2014;68:8–13. doi: 10.1038/ejcn.2013.177. [ DOI ] [ PubMed ] [ Google Scholar ]
- 79. Reynolds A., Mann J., Cummings J., Winter N., Mete E., Te Morenga L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet. 2019;393:434–445. doi: 10.1016/S0140-6736(18)31809-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 80. Fung T.T., Schulze M., Manson J.E., Willett W.C., Hu F.B. Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch. Intern. Med. 2004;164:2235–2240. doi: 10.1001/archinte.164.20.2235. [ DOI ] [ PubMed ] [ Google Scholar ]
- 81. Salas-Salvado J., Guasch-Ferre M., Lee C.H., Estruch R., Clish C.B., Ros E. Protective Effects of the Mediterranean Diet on Type 2 Diabetes and Metabolic Syndrome. J. Nutr. 2016;146:920–927. doi: 10.3945/jn.115.218487. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 82. Jannasch F., Kroger J., Schulze M.B. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J. Nutr. 2017;147:1174–1182. doi: 10.3945/jn.116.242552. [ DOI ] [ PubMed ] [ Google Scholar ]
- 83. Tonstad S., Stewart K., Oda K., Batech M., Herring R.P., Fraser G.E. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr. Metab. Cardiovasc. Dis. 2013;23:292–299. doi: 10.1016/j.numecd.2011.07.004. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 84. Snowdon D.A., Phillips R.L. Does a vegetarian diet reduce the occurrence of diabetes? Am. J. Public Health. 1985;75:507–512. doi: 10.2105/AJPH.75.5.507. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 85. Chiu T.H.T., Pan W.H., Lin M.N., Lin C.L. Vegetarian diet, change in dietary patterns, and diabetes risk: A prospective study. Nutr. Diabetes. 2018;8:12. doi: 10.1038/s41387-018-0022-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 86. Zhang C., Tobias D.K., Chavarro J.E., Bao W., Wang D., Ley S.H., Hu F.B. Adherence to healthy lifestyle and risk of gestational diabetes mellitus: Prospective cohort study. BMJ. 2014;349:g5450. doi: 10.1136/bmj.g5450. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 87. Mekary R.A., Giovannucci E., Willett W.C., van Dam R.M., Hu F.B. Eating patterns and type 2 diabetes risk in men: Breakfast omission, eating frequency, and snacking. Am. J. Clin. Nutr. 2012;95:1182–1189. doi: 10.3945/ajcn.111.028209. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 88. Mekary R.A., Giovannucci E., Cahill L., Willett W.C., van Dam R.M., Hu F.B. Eating patterns and type 2 diabetes risk in older women: Breakfast consumption and eating frequency. Am. J. Clin. Nutr. 2013;98:436–443. doi: 10.3945/ajcn.112.057521. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 89. Ballon A., Neuenschwander M., Schlesinger S. Breakfast Skipping Is Associated with Increased Risk of Type 2 Diabetes among Adults: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Nutr. 2019;149:106–113. doi: 10.1093/jn/nxy194. [ DOI ] [ PubMed ] [ Google Scholar ]
- 90. Lindstrom J., Peltonen M., Eriksson J.G., Louheranta A., Fogelholm M., Uusitupa M., Tuomilehto J. High-fibre, low-fat diet predicts long-term weight loss and decreased type 2 diabetes risk: The Finnish Diabetes Prevention Study. Diabetologia. 2006;49:912–920. doi: 10.1007/s00125-006-0198-3. [ DOI ] [ PubMed ] [ Google Scholar ]
- 91. Sylvetsky A.C., Edelstein S.L., Walford G., Boyko E.J., Horton E.S., Ibebuogu U.N., Knowler W.C., Montez M.G., Temprosa M., Hoskin M., et al. A High-Carbohydrate, High-Fiber, Low-Fat Diet Results in Weight Loss among Adults at High Risk of Type 2 Diabetes. J. Nutr. 2017;147:2060–2066. doi: 10.3945/jn.117.252395. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 92. Wu J.H.Y., Marklund M., Imamura F., Tintle N., Ardisson Korat A.V., de Goede J., Zhou X., Yang W.S., de Oliveira Otto M.C., Kröger J., et al. Omega-6 fatty acid biomarkers and incident type 2 diabetes: Pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017;5:965–974. doi: 10.1016/S2213-8587(17)30307-8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 93. Schwab U., Lauritzen L., Tholstrup T., Haldorssoni T., Riserus U., Uusitupa M., Becker W. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: A systematic review. Food Nutr. Res. 2014;58:25145. doi: 10.3402/fnr.v58.25145. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 94. Laaksonen D.E., Lindstrom J., Lakka T.A., Eriksson J.G., Niskanen L., Wikstrom K., Aunola S., Keinanen-Kiukaanniemi S., Laakso M., Valle T.T., et al. Physical activity in the prevention of type 2 diabetes: The Finnish diabetes prevention study. Diabetes. 2005;54:158–165. doi: 10.2337/diabetes.54.1.158. [ DOI ] [ PubMed ] [ Google Scholar ]
- 95. Aune D., Norat T., Leitzmann M., Tonstad S., Vatten L.J. Physical activity and the risk of type 2 diabetes: A systematic review and dose-response meta-analysis. Eur. J. Epidemiol. 2015;30:529–542. doi: 10.1007/s10654-015-0056-z. [ DOI ] [ PubMed ] [ Google Scholar ]
- 96. Burr J.F., Rowan C.P., Jamnik V.K., Riddell M.C. The role of physical activity in type 2 diabetes prevention: Physiological and practical perspectives. Phys. Sportsmed. 2010;38:72–82. doi: 10.3810/psm.2010.04.1764. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (2.3 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 06 June 2022
The burden and risks of emerging complications of diabetes mellitus
- Dunya Tomic ORCID: orcid.org/0000-0003-2471-2523 1 , 2 ,
- Jonathan E. Shaw ORCID: orcid.org/0000-0002-6187-2203 1 , 2 na1 &
- Dianna J. Magliano ORCID: orcid.org/0000-0002-9507-6096 1 , 2 na1
Nature Reviews Endocrinology volume 18 , pages 525–539 ( 2022 ) Cite this article
65k Accesses
371 Citations
55 Altmetric
Metrics details
- Diabetes complications
- Type 1 diabetes
- Type 2 diabetes
The traditional complications of diabetes mellitus are well known and continue to pose a considerable burden on millions of people living with diabetes mellitus. However, advances in the management of diabetes mellitus and, consequently, longer life expectancies, have resulted in the emergence of evidence of the existence of a different set of lesser-acknowledged diabetes mellitus complications. With declining mortality from vascular disease, which once accounted for more than 50% of deaths amongst people with diabetes mellitus, cancer and dementia now comprise the leading causes of death in people with diabetes mellitus in some countries or regions. Additionally, studies have demonstrated notable links between diabetes mellitus and a broad range of comorbidities, including cognitive decline, functional disability, affective disorders, obstructive sleep apnoea and liver disease, and have refined our understanding of the association between diabetes mellitus and infection. However, no published review currently synthesizes this evidence to provide an in-depth discussion of the burden and risks of these emerging complications. This Review summarizes information from systematic reviews and major cohort studies regarding emerging complications of type 1 and type 2 diabetes mellitus to identify and quantify associations, highlight gaps and discrepancies in the evidence, and consider implications for the future management of diabetes mellitus.
With advances in the management of diabetes mellitus, evidence is emerging of an increased risk and burden of a different set of lesser-known complications of diabetes mellitus.
As mortality from vascular diseases has declined, cancer and dementia have become leading causes of death amongst people with diabetes mellitus.
Diabetes mellitus is associated with an increased risk of various cancers, especially gastrointestinal cancers and female-specific cancers.
Hospitalization and mortality from various infections, including COVID-19, pneumonia, foot and kidney infections, are increased in people with diabetes mellitus.
Cognitive and functional disability, nonalcoholic fatty liver disease, obstructive sleep apnoea and depression are also common in people with diabetes mellitus.
As new complications of diabetes mellitus continue to emerge, the management of this disorder should be viewed holistically, and screening guidelines should consider conditions such as cancer, liver disease and depression.
Similar content being viewed by others
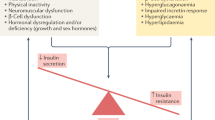
Type 2 diabetes mellitus in older adults: clinical considerations and management
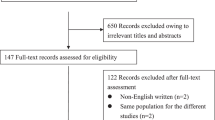
The cross-sectional and longitudinal relationship of diabetic retinopathy to cognitive impairment: a systematic review and meta-analysis

Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025
Introduction.
Diabetes mellitus is a common, albeit potentially devastating, medical condition that has increased in prevalence over the past few decades to constitute a major public health challenge of the twenty-first century 1 . Complications that have traditionally been associated with diabetes mellitus include macrovascular conditions, such as coronary heart disease, stroke and peripheral arterial disease, and microvascular conditions, including diabetic kidney disease, retinopathy and peripheral neuropathy 2 (Fig. 1 ). Heart failure is also a common initial manifestation of cardiovascular disease in patients with type 2 diabetes mellitus (T2DM) 3 and confers a high risk of mortality in those with T1DM or T2DM 4 . Although a great burden of disease associated with these traditional complications of diabetes mellitus still exists, rates of these conditions are declining with improvements in the management of diabetes mellitus 5 . Instead, as people with diabetes mellitus are living longer, they are becoming susceptible to a different set of complications 6 . Population-based studies 7 , 8 , 9 show that vascular disease no longer accounts for most deaths among people with diabetes mellitus, as was previously the case 10 . Cancer is now the leading cause of death in people with diabetes mellitus in some countries or regions (hereafter ‘countries/regions’) 9 , and the proportion of deaths due to dementia has risen since the turn of the century 11 . In England, traditional complications accounted for more than 50% of hospitalizations in people with diabetes mellitus in 2003, but for only 30% in 2018, highlighting the shift in the nature of complications of this disorder over this corresponding period 12 .
The traditional complications of diabetes mellitus include stroke, coronary heart disease and heart failure, peripheral neuropathy, retinopathy, diabetic kidney disease and peripheral vascular disease, as represented on the left-hand side of the diagram. With advances in the management of diabetes mellitus, associations between diabetes mellitus and cancer, infections, functional and cognitive disability, liver disease and affective disorders are instead emerging, as depicted in the right-hand side of the diagram. This is not an exhaustive list of complications associated with diabetes mellitus.
Cohort studies have reported associations of diabetes mellitus with various cancers, functional and cognitive disability, liver disease, affective disorders and sleep disturbance, and have provided new insights into infection-related complications of diabetes mellitus 13 , 14 , 15 , 16 , 17 . Although emerging complications have been briefly acknowledged in reviews of diabetes mellitus morbidity and mortality 11 , 17 , no comprehensive review currently specifically provides an analysis of the evidence for the association of these complications with diabetes mellitus. In this Review, we synthesize information published since the year 2000 on the risks and burden of emerging complications associated with T1DM and T2DM.
Diabetes mellitus and cancer
The burden of cancer mortality.
With the rates of cardiovascular mortality declining amongst people with diabetes mellitus, cancer deaths now constitute a larger proportion of deaths among this population in some countries/regions 8 , 9 . Although the proportion of deaths due to cancer appears to be stable, at around 16–20%, in the population with diabetes mellitus in the USA 7 , in England it increased from 22% to 28% between 2001 and 2018 (ref. 9 ), with a similar increase reported in Australia 8 . Notably, in England, cancer has overtaken vascular disease as the leading cause of death in people with diabetes mellitus and it is the leading contributor to excess mortality in those with diabetes mellitus compared with those without 9 . These findings are likely to be due to a substantial decline in the proportion of deaths from vascular diseases, from 44% to 24% between 2001 and 2018, which is thought to reflect the targeting of prevention measures in people with diabetes mellitus 18 . Over the same time period, cancer mortality rates fell by much less in the population with diabetes mellitus than in that without diabetes 9 , suggesting that clinical approaches for diabetes mellitus might focus too narrowly on vascular complications and might require revision 19 . In addition, several studies have reported that female patients with diabetes mellitus receive less-aggressive treatment for breast cancer compared with patients without diabetes mellitus, particularly with regard to chemotherapy 20 , 21 , 22 , suggesting that this treatment approach might result in increased cancer mortality rates in women with diabetes mellitus compared with those without diabetes mellitus. Although substantial investigation of cancer mortality in people with diabetes mellitus has been undertaken in high-income countries/regions, there is a paucity of evidence from low-income and middle-income countries/regions. It is important to understand the potential effect of diabetes mellitus on cancer mortality in these countries/regions owing to the reduced capacity of health-care systems in these countries/regions to cope with the combination of a rising prevalence of diabetes mellitus and rising cancer mortality rates in those with diabetes mellitus. One study in Mauritius showed a significantly increased risk of all-cause cancer mortality in patients with T2DM 23 , but this study has yet to be replicated in other low-income and middle-income countries/regions.
Gastrointestinal cancers
Of the reported associations between diabetes mellitus and cancer (Table 1 ), some of the strongest have been demonstrated for gastrointestinal cancers.
Hepatocellular carcinoma
In the case of hepatocellular carcinoma, the most rigorous systematic review on the topic — comprising 18 cohort studies with a combined total of more than 3.5 million individuals — reported a summary relative risk (SRR) of 2.01 (95% confidence interval (CI) 1.61–2.51) for an association with diabetes mellitus 24 . This increased risk of hepatocellular carcinoma with diabetes mellitus is supported by the results of another systematic review that included case–control studies 25 . Another review also found that diabetes mellitus independently increased the risk of hepatocellular carcinoma in the setting of hepatitis C virus infection 26 .
Pancreatic cancer
The risk of pancreatic cancer appears to be approximately doubled in patients with T2DM compared with patients without T2DM. A meta-analysis of 36 studies found an adjusted odds ratio (OR) of 1.82 (95% CI 1.66–1.89) for pancreatic cancer among people with T2DM compared with patients without T2DM 27 (Table 1 ). However, it is possible that these findings are influenced by reverse causality — in this scenario, diabetes mellitus is triggered by undiagnosed pancreatic cancer 28 , with pancreatic cancer subsequently being clinically diagnosed only after the diagnosis of diabetes mellitus. Nevertheless, although the greatest risk (OR 2.05, 95% CI 1.87–2.25) of pancreatic cancer was seen in people diagnosed with T2DM 1–4 years previously compared with people without T2DM, those with a diagnosis of T2DM of more than 10 years remained at increased risk of pancreatic cancer (OR 1.51, 95% CI 1.16–1.96) 27 , suggesting that reverse causality can explain only part of the association between T2DM and pancreatic cancer. Although T2DM accounts for ~90% of all cases of diabetes mellitus 29 , a study incorporating data from five nationwide diabetes registries also reported an increased risk of pancreatic cancer amongst both male patients (HR 1.53, 95% CI 1.30–1.79) and female patients (HR 1.25, 95% CI 1.02–1.53) with T1DM 30 .
Colorectal cancer
For colorectal cancer, three systematic reviews have shown a consistent 20–30% increased risk associated with diabetes mellitus 31 , 32 , 33 . One systematic review, which included more than eight million people across 30 cohort studies, reported an incidence SRR of 1.27 (95% CI 1.21–1.34) of colorectal cancer 31 , independent of sex and family history (Table 1 ). Similar increases in colorectal cancer incidence in patients with diabetes mellitus were reported in a meta-analysis of randomized controlled trials (RCTs) and cohort studies 32 and in a systematic review that included cross-sectional studies 33 .
Female-specific cancers
Endometrial, breast and ovarian cancers all occur more frequently in women with diabetes mellitus than in women without diabetes mellitus.
Endometrial cancer
For endometrial cancer, one systematic review of 29 cohort studies and a combined total of 5,302,259 women reported a SRR of 1.89 (95% CI 1.46–2.45) and summary incidence rate ratio (IRR) of 1.61 (95% CI 1.51–1.71) 34 (Table 1 ). Similar increased risks were found in two systematic reviews incorporating cross-sectional studies 35 , 36 , one of which found a particularly strong association of T1DM (relative risk (RR) 3.15, 95% CI 1.07–9.29) with endometrial cancer.
Breast cancer
The best evidence for a link between diabetes mellitus and breast cancer comes from a systematic review of six prospective cohort studies and more than 150,000 women, in which the hazard ratio (HR) for the incidence of breast cancer in women with diabetes mellitus compared with women without diabetes mellitus was 1.23 (95% CI 1.12–1.34) 32 (Table 1 ). Two further systematic reviews have also shown this increased association 37 , 38 .
The association of diabetes mellitus with breast cancer appears to vary according to menopausal status. In a meta-analysis of studies of premenopausal women with diabetes mellitus, no significant association with breast cancer was found 39 , whereas in 11 studies that included only postmenopausal women, the SRR was 1.15 (95% CI 1.07–1.24). The difference in breast cancer risk between premenopausal and postmenopausal women with diabetes mellitus was statistically significant. The increased risk of breast cancer after menopause in women with diabetes mellitus compared with women without diabetes mellitus might result from the elevated concentrations and increased bioavailability of oestrogen that are associated with adiposity 40 , which is a common comorbidity in those with T2DM; oestrogen synthesis occurs in adipose tissue in postmenopausal women, while it is primarily gonadal in premenopausal women 41 . Notably, however, there is evidence that hormone-receptor-negative breast cancers, which typically carry a poor prognosis, occur more frequently in women with breast cancer and diabetes mellitus than in women with breast cancer and no diabetes mellitus 42 , indicating that non-hormonal mechanisms also occur.

Ovarian cancer
Diabetes mellitus also appears to increase the risk of ovarian cancer, with consistent results from across four systematic reviews. A pooled RR of 1.32 (95% CI 1.14–1.52) was reported across 15 cohort studies and a total of more than 2.3 million women 43 (Table 1 ). A SRR of 1.19 (95% CI 1.06–1.34) was found across 14 cohort studies and 3,708,313 women 44 . Similar risks were reported in meta-analyses that included cross-sectional studies 45 , 46 .
Male-specific cancers: prostate cancer
An inverse association between diabetes mellitus and prostate cancer has been observed in a systematic review (RR 0.91, 95% CI 0.86–0.96) 47 , and is probably due to reduced testosterone levels that occur secondary to the low levels of sex hormone-binding globulin that are commonly seen in men with T2DM and obesity 48 . Notably, however, the systematic review that showed the inverse association involved mostly white men (Table 1 ), whereas a systematic review of more than 1.7 million men from Taiwan, Japan, South Korea and India found that diabetes mellitus increased prostate cancer risk 49 , suggesting that ethnicity might be an effect modifier of the diabetes mellitus–prostate cancer relationship. The mechanisms behind this increased risk in men in regions of Asia such as Taiwan and Japan, where most study participants came from, remain unclear. Perhaps, as Asian men develop diabetes mellitus at lower levels of total adiposity than do white men 50 , the adiposity associated with diabetes mellitus in Asian men might have a lesser impact on sex hormone-binding globulin and testosterone than it does in white men. Despite the reported inverse association between diabetes mellitus and prostate cancer in white men, however, evidence suggests that prostate cancers that do develop in men with T2DM are typically more aggressive, conferring higher rates of disease-specific mortality than prostate cancers in men without diabetes mellitus 51 .
An assessment of cancer associations
As outlined above, a wealth of data has shown that diabetes mellitus is associated with an increased risk of various cancers. It has been argued, however, that some of these associations could be due to detection bias resulting from increased surveillance of people with diabetes mellitus in the immediate period after diagnosis 52 , or reverse causality, particularly in the case of pancreatic cancer 53 . However, neither phenomenon can account for the excess risks seen in the longer term. An Australian study exploring detection bias and reverse causality found that standardized mortality ratios (SMRs) for several cancer types in people with diabetes mellitus compared with the general population fell over time, but remained elevated beyond 2 years for pancreatic and liver cancers 54 , suggesting that diabetes mellitus is a genuine risk factor for these cancer types.
A limitation of the evidence that surrounds diabetes mellitus and cancer risk is high clinical and methodological heterogeneity across several of the large systematic reviews, which makes it difficult to be certain of the effect size in different demographic groups. Additionally, many of the studies exploring a potential association between diabetes mellitus and cancer were unable to adjust for BMI, which is a major confounder. However, a modelling study that accounted for BMI found that although 2.1% of cancers worldwide in 2012 were attributable to diabetes mellitus as an independent risk factor, twice as many cancers were attributable to high BMI 55 , so it is likely that effect sizes for cancer risk associated with diabetes mellitus would be attenuated after adjustment for BMI. Notably, however, low-income and middle-income countries/regions had the largest increase in the numbers of cases of cancer attributable to diabetes mellitus both alone and in combination with BMI 55 , highlighting the need for public health intervention, given that these countries/regions are less equipped than high-income countries/regions to manage a growing burden of cancer.
As well as the cancer types outlined above, diabetes mellitus has also been linked to various other types of cancer, including kidney cancer 56 , bladder cancer 57 and haematological malignancies; however, the evidence for these associations is not as strong as for the cancers discussed above 58 . Diabetes mellitus might also be associated with other cancer types such as small intestine cancer, but the rarity of some of these types makes it difficult to obtain sufficient statistical power in analyses of any potential association.
Potential aetiological mechanisms
Several aetiological mechanisms that might be involved in linking diabetes mellitus to cancer have been proposed, including hyperinsulinaemia, hyperglycaemia, inflammation and cellular signalling mechanisms.
Hyperinsulinaemia
Most cancer cells express insulin receptors, through which hyperinsulinaemia is thought to stimulate cancer cell proliferation and metastasis 59 . Hyperinsulinaemia might also promote carcinogenesis through increased local levels of insulin-like growth factor 1 (IGF1), which has potent mitogenic and anti-apoptotic activities 60 , owing to decreased levels of insulin-like growth factor binding proteins. As outlined above, people with diabetes mellitus show a strong risk of pancreatic and liver cancers; this increased risk might occur because insulin is produced by pancreatic β-cells and transported to the liver via the portal vein 61 , thereby exposing the liver and pancreas to high levels of endogenous insulin 59 .
Hyperglycaemia and inflammation
Hyperglycaemia can induce DNA damage 62 , increase the generation of reactive oxygen species 63 and downregulate antioxidant expression 64 , all of which are associated with cancer development. Inflammatory markers, including cytokines such as IL-6, appear to have an important role in the association between diabetes and cancer 65 .
Cellular signalling mechanisms
Several cellular signalling components are common to the pathogenesis of T2DM and cancer. These include the mechanistic target of rapamycin (mTOR), a central controller of cell growth and proliferation; AMP-activated protein kinase, a cellular energy sensor and signal transducer 66 ; and the phosphatidylinositol 3-kinase (PI3K)–AKT pathway, which transduces growth factor signals during organismal growth, glucose homeostasis and cell proliferation 67 . Dysregulation of any of these cellular signalling components or pathways could contribute to the development of cancer and metabolic disorders, including T2DM, and glucose-lowering drugs such as metformin have been associated with a reduction in cancer cell proliferation through effective inhibition of some of these components 68 .
Diabetes mellitus and infections
Infection-related complications.
Although infection has long been recognized as a complication of diabetes mellitus, an association between diabetes mellitus and infection has not been well documented in epidemiological studies 69 . Only in the past decade have major studies quantified the burden of infection-related complications in people with diabetes mellitus and explored the specific infections accounting for this burden. In a US cohort of 12,379 participants, diabetes mellitus conferred a significant risk of infection-related hospitalization, with an adjusted HR of 1.67 (95% CI 1.52–1.83) compared with people without diabetes mellitus 70 (Table 2 ). The association was most pronounced for foot infections (HR 5.99, 95% CI 4.38–8.19), with significant associations also observed for respiratory infection, urinary tract infection, sepsis and post-operative infection, but not for gastrointestinal infection, a category that included appendicitis and gastrointestinal abscesses but not viral or bacterial gastroenteritis. Interestingly, a report from Taiwan demonstrated an association between the use of metformin and a lower risk of appendicitis 71 .
In an analysis of the entire Hong Kong population over the period 2001–2016, rates of hospitalization for all types of infection remained consistently higher in people with diabetes mellitus than in those without diabetes mellitus 72 . The strongest association was seen for hospitalization due to kidney infections, for which the adjusted RR was 4.9 (95% CI 3.9–6.2) in men and 3.2 (95% CI 2.8–3.7) in women with diabetes mellitus compared with those without diabetes mellitus in 2016 (Table 2 ). Diabetes mellitus roughly doubled the risk of hospitalization from tuberculosis or sepsis. The most common cause of infection-related hospitalization was pneumonia, which accounted for 39% of infections across the study period, while no other single cause accounted for more than 25% of infections across the same period. Pneumonia-related hospitalization rates increased substantially from 2001 to 2005, probably as a result of the 2003 severe acute respiratory syndrome (SARS) epidemic and the decreased threshold for pneumonia hospitalization in the immediate post-epidemic period. Rates for hospitalization for influenza increased from 2002 to 2016, possibly because of changes in the virus and increased testing for influenza. Declining rates of hospitalization for tuberculosis, urinary tract infections, foot infections and sepsis could be due to improvements in the management of diabetes mellitus.
Infection-related mortality rates were found to be significantly elevated among 1,108,982 Australians with diabetes mellitus studied over the period 2000–2010 compared with rates in people without diabetes mellitus 73 . For overall infection-related mortality, SMRs were 4.42 (95% CI 3.68–5.34) for T1DM and 1.47 (95% CI 1.42–1.53) for people with T2DM compared with those without diabetes mellitus (Table 2 ). Substantially higher infection-related mortality rates were seen in people with T1DM compared with those with T2DM for all infection types, even after accounting for age. Hyperglycaemia is thought to be a driver of infection amongst people with diabetes mellitus (see below) 73 , which might explain the higher SMRs amongst people with T1DM, in whom hyperglycaemia is typically more severe, than in those with T2DM. The highest SMRs were seen for osteomyelitis, and SMRs for septicaemia and pneumonia were also greater than 1.0 for both types of diabetes mellitus compared with those without diabetes mellitus.
Post-operative infection
Post-operative infection is also an important complication of diabetes mellitus. In a meta-analysis, diabetes mellitus was found to be associated with an OR of 1.77 (95% CI 1.13–2.78) for surgical site infection across studies that adjusted for confounding factors 74 (Table 2 ). The effect size appears to be greatest after cardiac procedures, and one US study of patients undergoing coronary artery bypass grafting found diabetes mellitus to be an independent predictor of surgical site infection, with an OR of 4.71 (95% CI 2.39–9.28) compared with those without diabetes mellitus 75 . Risks of infection of more than threefold were reported in some studies of gynaecological 76 and spinal surgery 77 in people with diabetes mellitus compared with those without diabetes mellitus. Increased risks of infection among people with diabetes mellitus were also observed in studies of colorectal and breast surgery and arthroplasty, suggesting that the association between diabetes mellitus and post-operative infection is present across a wide range of types of surgery 74 .
Respiratory infections
The incidence of hospitalizations due to respiratory infections among people with diabetes mellitus was increasing substantially even before the onset of the coronavirus disease 2019 (COVID-19) pandemic, probably owing to increased life expectancy in these patients as well as an increased likelihood of them being hospitalized for conditions such as respiratory infections, which occur mostly in older age 12 . This rising burden of respiratory infection, in combination with the rising prevalence of diabetes mellitus, highlights the importance of addressing the emerging complications of diabetes mellitus to minimize impacts on health-care systems in current and future global epidemics.
Although diabetes mellitus does not appear to increase the risk of becoming infected with COVID-19 (ref. 78 ), various population-based studies have reported increased risks of COVID-19 complications among people with diabetes mellitus. In a study of the total Scottish population, people with diabetes mellitus were found to have an increased risk of fatal or critical care unit-treated COVID-19, with an adjusted OR of 1.40 (95% CI 1.30–1.50) compared with those without diabetes mellitus 79 (Table 2 ). The risk was particularly high for those with T1DM (OR 2.40, 95% CI 1.82–3.16) 79 . Both T1DM and T2DM have been linked to a more than twofold increased risk of hospitalization with COVID-19 in a large Swedish cohort study 80 . In South Korean studies, T2DM was linked to intensive care unit admission among patients with COVID-19 infection 81 , and diabetes mellitus (either T1DM or T2DM) was linked to a requirement for ventilation and oxygen therapy 82 in patients with COVID-19. Diabetes mellitus appears to be the primary predisposing factor for opportunistic infection with mucormycosis in individuals with COVID-19 (ref. 83 ). The evidence for diabetes mellitus as a risk factor for post-COVID-19 syndrome is inconclusive 84 , 85 . Interestingly, an increase in the incidence of T1DM during the COVID-19 pandemic has been reported in several countries/regions 86 , and some data suggest an increased risk of T1DM after COVID-19 infection 87 , but the evidence regarding a causal effect is inconclusive.
Pneumonia, MERS, SARS and H1N1 influenza
The data regarding diabetes mellitus and COVID-19 are consistent with the published literature regarding other respiratory infections, such as pneumonia, for which diabetes mellitus has been shown to increase the risk of hospitalization 88 and mortality 88 , with similar effect sizes to those seen for COVID-19, compared with no diabetes mellitus. Diabetes mellitus has also been also linked to adverse outcomes in people with Middle East respiratory syndrome (MERS), SARS and H1N1 influenza 89 , 90 , 91 , 92 , suggesting that mechanisms specific to COVID-19 are unlikely to be responsible for the relationship between diabetes mellitus and COVID-19. Unlike the case for COVID-19, there is evidence that people with diabetes mellitus are at increased risk of developing certain other respiratory infections, namely pneumonia 93 and possibly also MERS 94 .
The mechanisms that might link diabetes mellitus and infection include a reduced T cell response, reduced neutrophil function and disorders of humoral immunity.
Mononuclear cells and monocytes of individuals with diabetes mellitus secrete less IL-1 and IL-6 than the same cells from people without diabetes mellitus 95 . The release of IL-1 and IL-6 by T cells and other cell types in response to infection has been implicated in the response to several viral infections 96 . Thus, the reduced secretion of these cytokines in patients with diabetes mellitus might be associated with the poorer responses to infection observed among these patients compared with people without diabetes mellitus.
In the context of neutrophil function, hyperglycaemic states might give rise to reductions in the mobilization of polymorphonuclear leukocytes, phagocytic activity and chemotaxis 97 , resulting in a decreased immune response to infection. Additionally, increased levels of glucose in monocytes isolated from patients with obesity and/or diabetes mellitus have been found to promote viral replication in these cells, as well as to enhance the expression of several cytokines, including pro-inflammatory cytokines that are associated with the COVID-19 ‘cytokine storm’; furthermore, glycolysis was found to sustain the SARS coronavirus 2 (SARS-CoV-2)-induced monocyte response and viral replication 98 .
Elevated glucose levels in people with diabetes mellitus are also associated with an increase in glycation, which, by promoting a change in the structure and/or function of several proteins and lipids, is responsible for many of the complications of diabetes mellitus 99 . In people with diabetes mellitus, antibodies can become glycated, a process that is thought to impair their biological function 100 . Although the clinical relevance of this impairment is not clear, it could potentially explain the results of an Israeli study that reported reduced COVID-19 vaccine effectiveness among people with T2DM compared with those without T2DM 101 .
Diabetes mellitus and liver disease
Nonalcoholic fatty liver disease.
The consequences of nonalcoholic fatty liver disease (NAFLD) make it important to recognize the burden of this disease among people with diabetes mellitus. NAFLD and nonalcoholic steatohepatitis (NASH; an advanced form of NAFLD) are major causes of liver transplantation in the general population. In the USA, NASH accounted for 19% of liver transplantations in 2016 — second only to alcoholic liver disease, which was the cause of 24% of transplantations 102 . In Australia and New Zealand, NAFLD was the primary diagnosis in 9% of liver transplant recipients in 2019, only slightly below the figure for alcoholic cirrhosis of 13% 103 . In Europe, NASH increased as the reason for transplantations from 1% in 2002 to more than 8% in 2016, in parallel with the rising prevalence of diabetes mellitus 104 .
NAFLD is highly prevalent among people with T2DM. In a systematic review of 80 studies across 20 countries/regions, the prevalence of NAFLD among 49,419 people with T2DM was 56% 105 , while the global prevalence of NAFLD in the general population is estimated to be 25% 106 . In a Chinese cohort study of 512,891 adults, diabetes mellitus was associated with an adjusted HR of 1.76 (95% CI 1.47–2.16) for NAFLD compared with no diabetes mellitus 107 (Table 3 ). Another smaller longitudinal Chinese study also reported an increased risk of developing NAFLD among those with T2DM compared with those without T2DM 108 . However, most evidence regarding the association between NAFLD and diabetes mellitus is from cross-sectional studies 109 , 110 , 111 .
NASH and fibrosis
Diabetes mellitus appears to enhance the risk of NAFLD complications, including NASH and fibrosis. An analysis of 892 people with NAFLD and T2DM across 10 studies showed that the prevalence of NASH was 37% (ref. 105 ); figures for the prevalence of NASH in the general population with NAFLD vary greatly across different study populations, ranging from 16% to 68% 112 . Amongst 439 people with T2DM and NAFLD in seven studies, 17% had advanced fibrosis 105 . An analysis of 1,069 people with NAFLD in a US study found that diabetes mellitus was an independent predictor for NASH (OR 1.93, 95% CI 1.37–2.73) and fibrosis (3.31, 95% CI 2.26–4.85) 113 .
Bidirectional relationship between diabetes mellitus and liver disease
The relationship between diabetes mellitus and NAFLD is bidirectional, as NAFLD is associated with an increased risk of developing T2DM 114 . There is also a notable bidirectional relationship between diabetes mellitus and liver cirrhosis. The prevalence of diabetes mellitus in people with liver cirrhosis has been reported as 20–63%, depending on the severity of liver damage, aetiology and diagnostic criteria 115 . In an Italian study of 401 participants with cirrhosis, 63% of those with decompensated liver disease had diabetes mellitus compared with 10% of those with well-compensated liver disease 116 , suggesting that diabetes mellitus is more common in severe cases of liver damage. The association between diabetes mellitus and cirrhosis also varies according to the cause of liver disease. In a US study of 204 people with cirrhosis, the prevalence of diabetes mellitus was 25% among those with cirrhosis caused by hepatitis C virus, 19% among those with cirrhosis from alcoholic liver disease and only 1% among those with cirrhosis due to cholestatic liver disease 117 . Among the causes of cirrhosis, haemochromatosis has the strongest association with diabetes mellitus, with diabetes mellitus mainly resulting from the iron deposition that is characteristic of haemochromatosis 118 .
Several factors have been implicated in the aetiology of liver disease in people with diabetes mellitus, with insulin resistance being the most notable 119 .
Insulin resistance
Insulin resistance causes lipolysis, thereby increasing the circulating levels of free fatty acids, which are then taken up by the liver as an energy source 120 . These fatty acids overload the mitochondrial β-oxidation system in the liver, resulting in the accumulation of fatty acids and, consequently, NAFLD 121 . Of those individuals with NAFLD, 2–3% develop hepatic inflammation, necrosis and fibrosis, which are the hallmarks of NASH 122 . The exact mechanisms leading to steatohepatitis are unclear, although dysregulated peripheral lipid metabolism appears to be important 14 .
Ectopic adipose deposition
Excessive or ectopic deposition of adipose tissue around the viscera and in the liver might be an important mechanism underlying both T2DM and liver disease, particularly NAFLD 123 . Dysfunction of long-term adipose storage in white adipose tissue is known to lead to ectopic adipose deposition in the liver. In this state, increased levels of fatty acyl-coenzyme As, the activated form of fatty acids, might lead to organ dysfunction, including NAFLD 124 . Ectopic adipose deposition leading to organ-specific insulin resistance has emerged as a major hypothesis for the pathophysiological basis of T2DM, and ectopic adipose in the pancreas could contribute to β-cell dysfunction and, thus, the development of T2DM 125 .
Diabetes mellitus and affective disorders
The prevalence of depression appears to be high among people with diabetes mellitus. The strongest evidence for an association comes from a systematic review of 147 studies among people with T2DM, which revealed a mean prevalence of depression of 28% 126 , while the global prevalence of depression in the general population is estimated at around 13% 127 . For T1DM, a systematic review reported a pooled prevalence of depression of 12% compared with only 3% in those without T1DM 128 . The risk of depression among people with diabetes mellitus appears to be roughly 25% greater than the risk in the general population, with consistent findings across several meta-analyses (Table 4 ). A 2013 study found an adjusted RR of 1.25 (95% CI 1.10–1.44) for incident depression among people with diabetes mellitus compared with those without diabetes mellitus 129 . Another systematic review of people with T2DM reported a near identical effect size 130 .
Anxiety and eating disorders
Evidence exists for an association of diabetes mellitus with anxiety, and of T1DM with eating disorders. In a systematic review involving 2,584 individuals with diabetes mellitus, a prevalence of 14% was found for generalized anxiety disorder and 40% for anxiety symptoms, whereas the prevalence of generalized anxiety disorder in the general population is estimated as only 3–4% 131 . People with diabetes mellitus had an increased risk of anxiety disorders (OR 1.20, 95% CI 1.10–1.31) and anxiety symptoms (OR 1.48, 95% CI 1.02–1.93) compared with those without diabetes mellitus in a meta-analysis 132 (Table 4 ), although these findings were based on cross-sectional data. Across 13 studies, 7% of adolescents with T1DM were found to have eating disorders, compared with 3% of peers without diabetes mellitus 133 .
Broader psychological impacts
There is a substantial literature on a broad range of psychological impacts of diabetes mellitus. Social stigma 134 can have profound impacts on the quality of life of not only people with diabetes mellitus, but their families and carers, too 135 . In a systematic review, diabetes mellitus distress was found to affect around one-third of adolescents with T1DM, which was consistent with the results of studies of adults with diabetes mellitus 136 . Diabetes mellitus burnout appears to be a distinct concept, and is characterized by exhaustion and detachment, accompanied by the experience of a loss of control over diabetes mellitus 137 .
Diabetes mellitus and depression appear to have common biological origins. Activation of the innate immune system and acute-phase inflammation contribute to the pathogenesis of T2DM — increased levels of inflammatory cytokines predict the onset of T2DM 138 — and there is growing evidence implicating cytokine-mediated inflammation in people with depression in the absence of diabetes mellitus 139 . Dysregulation of the hypothalamic–pituitary–adrenal axis is another potential biological mechanism linking depression and diabetes mellitus 140 . There have been numerous reports of hippocampal atrophy, which might contribute to chronic activation of the hypothalamic–pituitary–adrenal axis, in individuals with T2DM as well as those with depression 141 , 142 . A meta-analysis found that, although hypertension modified global cerebral atrophy in those with T2DM, it had no effect on hippocampal atrophy 143 . This suggests that, although global cerebral atrophy in individuals with T2DM might be driven by atherosclerotic disease, hippocampal atrophy is an independent effect that provides a common neuropathological aetiology for the comorbidity of T2DM with depression. There is a lack of relevant information regarding the potential aetiological mechanisms that link diabetes to other affective disorders.
Diabetes mellitus and sleep disturbance
Obstructive sleep apnoea.
Obstructive sleep apnoea (OSA) is highly prevalent among people with diabetes mellitus. In a systematic review of 41 studies of adults with diabetes mellitus, the prevalence of OSA was found to be 60% 144 , whereas reports for OSA prevalence in the general population range from 9% to 38% 145 . In a UK study of 1,656,739 participants, T2DM was associated with an IRR for OSA of 1.48 (95% CI 1.42–1.55) compared with no T2DM 146 . A population-based US study reported a HR of 1.53 (95% CI 1.32–1.77) for OSA in people with T2DM compared with those without diabetes mellitus 147 . However, the association in this latter report was attenuated after adjustment for BMI and waist circumference (1.08, 95% CI 1.00–1.16), suggesting that the excess risk of OSA among people with diabetes mellitus might be mainly explained by the comorbidity of obesity. Although most studies on OSA have focused on T2DM, a meta-analysis of people with T1DM revealed a similar prevalence of 52% 148 ; however, this meta-analysis was limited to small studies. The association between T2DM and OSA is bidirectional: the severity of OSA was shown to be positively associated with the incidence of T2DM, independent of adiposity, in a large US cohort study 149 .
The mechanism by which T2DM might increase the risk of developing OSA is thought to involve dysregulation of the autonomic nervous system leading to sleep-disordered breathing 150 . Conversely, the specific mechanism behind OSA as a causative factor for T2DM remains poorly understood. It has been suggested that OSA is able to induce insulin resistance 151 , 152 and is a risk factor for the development of glucose intolerance 152 . However, once T2DM has developed, there is no clear evidence that OSA worsens glycaemic control, as an RCT of people with T2DM found that treating OSA had no effect on glycaemic control 153 .
Diabetes mellitus and cognitive disability
Dementia and cognitive impairment.
Dementia is emerging as a major cause of mortality in both individuals with diabetes mellitus and the general population, and is now the leading cause of death in some countries/regions 9 . However, compared with the general population, diabetes mellitus increases the risk of dementia, particularly vascular dementia. The association is supported by several systematic reviews, including one of eight population-based studies with more than 23,000 people, which found SRRs of 2.38 (95% CI 1.79–3.18) for vascular dementia and 1.39 (95% CI 1.16–1.66) for Alzheimer disease comparing people with diabetes mellitus with those without diabetes mellitus 154 (Table 4 ). Similar results, as well as a RR of 1.21 (95% CI 1.02–1.45) for mild cognitive impairment (MCI), were reported across 19 population-based studies of 44,714 people, 6,184 of whom had diabetes mellitus 155 . Two meta-analyses of prospective cohort studies have shown increased risks of all-cause dementia in people with diabetes mellitus compared with those without diabetes mellitus 156 , 157 , and T2DM has been shown to increase progression to dementia in people with MCI 158 .
The boundaries between Alzheimer disease and vascular dementia remain controversial, and these conditions are often difficult to differentiate clinically 159 . Consequently, vascular dementia might have been misdiagnosed as Alzheimer disease in some studies investigating diabetes mellitus and dementia, resulting in an overestimation of the effect size of the association between diabetes mellitus and Alzheimer disease. Although a cohort study found a significant association between diabetes mellitus and Alzheimer disease using imaging 160 , autopsy studies have failed to uncover an association between diabetes mellitus and Alzheimer disease pathology 161 , 162 , suggesting that vascular mechanisms are the key driver of cognitive decline in people with diabetes mellitus.
Another important finding is a 45% prevalence of MCI among people with T2DM in a meta-analysis, compared with a prevalence of 3–22% reported for the general population 163 . Notably, however, the prevalence of MCI in individuals with T2DM was similar in people younger than 60 years (46%) and those older than 60 years (44%), which is at odds with previous research suggesting that MCI is most common in older people, particularly those aged more than 65 years 164 However, another meta-analysis found cognitive decline in people with T2DM who are younger than 65 years 165 , suggesting that a burden of cognitive disease exists among younger people with diabetes mellitus.
Although there is solid evidence that links diabetes mellitus to cognitive disability, our understanding of the underlying mechanisms is incomplete. Mouse models suggest a strong association between hyperglycaemia, the advanced glycation end products glyoxal and methylglyoxal, enhanced blood–brain barrier (BBB) permeability and cognitive dysfunction in both T1DM and T2DM 166 . The BBB reduces the access of neurotoxic compounds and pathogens to the brain and sustains brain homeostasis, so disruption to the BBB can result in cognitive dysfunction through dysregulation of transport of molecules between the peripheral circulation and the brain 167 . There appears to be a continuous relationship between glycaemia and cognition, with associations found between even high-normal blood levels of glucose and cognitive decline 168 . Another hypothetical mechanism involves a key role for impaired insulin signalling in the pathogenesis of Alzheimer disease. Brain tissue obtained post mortem from individuals with Alzheimer disease showed extensive abnormalities in insulin and insulin-like growth factor signalling mechanisms compared with control brain tissue 169 . Although the synthesis of insulin-like growth factors occurred normally in people with Alzheimer disease, their expression levels were markedly reduced, which led to the subsequent proposal of the term ‘type 3 diabetes’ to characterize Alzheimer disease.
Diabetes mellitus and disability
Functional disability.
Disability (defined as a difficulty in functioning in one or more life domains as experienced by an individual with a health condition in interaction with contextual factors) 170 is highly prevalent in people with diabetes mellitus. In a systematic review, lower-body functional limitation was found to be the most prevalent disability (47–84%) among people with diabetes mellitus 171 The prevalence of difficulties with activities of daily living among people with diabetes mellitus ranged from 12% to 55%, although most studies were conducted exclusively in individuals aged 60 years and above, so the results are not generalizable to younger age groups. A systematic review showed a significant association between diabetes mellitus and falls in adults aged 60 years and above 172 . A 2013 meta-analysis 173 showed an increased risk of mobility disability, activities of daily living disability and independent activities of daily living disability among people with diabetes mellitus compared with those without diabetes mellitus (Table 4 ). Although this analysis included cross-sectional data, results were consistent across longitudinal and cross-sectional studies, suggesting little effect of reverse causality. However, people with functional disabilities that limit mobility (for example, people with osteoarthritis or who have had a stroke) might be more prone to developing diabetes mellitus owing to physical inactivity 174 .
Workplace productivity
Decreased productivity while at work, increased time off work and early dropout from the workforce 175 are all associated with diabetes mellitus, probably partly due to functional disability, and possibly also to comorbidities such as obesity and physical inactivity 176 . Given that young-onset diabetes is becoming more common, and most people with diabetes mellitus in middle-income countries/regions are less than 65 years old 177 , a pandemic of diabetes mellitus-related work disability among a middle-aged population does not bode well for the economies of these regions.
The mechanisms by which diabetes mellitus leads to functional disability remain unclear. One suggestion is that hyperglycaemia leads to systemic inflammation, which is one component of a multifactorial process that results in disability 154 . The rapid loss of skeletal muscle strength and quality seen among people with diabetes mellitus might be another cause of functional disability 178 (Box 1 ). In addition, complications of diabetes mellitus, including stroke, peripheral neuropathy and cardiac dysfunction, can obviously directly cause disability 179 .
Box 1 Diabetes mellitus and skeletal muscle atrophy
Individuals with diabetes mellitus exhibit skeletal muscle atrophy that is typically mild in middle age and becomes more substantial with increasing age.
This muscle loss leads to reduced strength and functional capacity and, ultimately, increased mortality.
Skeletal muscle atrophy results from a negative balance between the rate of synthesis and degradation of contractile proteins, which occurs in response to disuse, ageing and chronic diseases such as diabetes mellitus.
Degradation of muscle proteins is more rapid in diabetes mellitus, and muscle protein synthesis has also been reported to be decreased.
Proposed mechanisms underlying skeletal muscle atrophy include systemic inflammation (affecting both protein synthesis and degradation), dysregulation of muscle protein anabolism and lipotoxicity.
Mouse models have also revealed a key role for the WWP1/KLF15 pathway, mediated by hyperglycaemia, in the pathogenesis of muscle atrophy.
See refs 195 , 196 , 197 , 198 .
Diabetes management and control
Although a detailed discussion of the impacts of anti-diabetes mellitus medications and glucose control on emerging complications is beyond the scope of this Review, their potential effect on these complications must be acknowledged.
Medications
Anti-diabetes mellitus medications and cancer.
In the case of cancer as an emerging complication, the use of medications for diabetes mellitus was not controlled for in most studies of diabetes mellitus and cancer and might therefore be a confounding factor. People taking metformin have a lower cancer risk than those not taking metformin 180 . However, this association is mainly accounted for by other factors. For example, metformin is less likely to be administered to people with diabetes mellitus who have kidney disease 181 , who typically have longer duration diabetes mellitus, which increases cancer risk. A review of observational studies into the association between metformin and cancer found that many studies reporting significant reductions in cancer incidence or mortality associated with metformin were affected by immortal time bias and other time-related biases, casting doubt on the ability of metformin to reduce cancer mortality 182 . Notably, the use of insulin was associated with an increased risk of several cancers in a meta-analysis 183 . However, in an RCT of more than 12,000 people with dysglycaemia, randomization to insulin glargine (compared with standard care) did not increase cancer incidence 184 . Furthermore, cancer rates in people with T1DM and T2DM do not appear to vary greatly, despite substantial differences in insulin use between people with these types of diabetes mellitus.
Anti-diabetes mellitus medications and other emerging complications
Anti-diabetes medications appear to affect the onset and development of some other emerging complications of diabetes mellitus. Results from RCTs suggest that metformin might confer therapeutic effects against depression 185 , and its use was associated with reduced dementia incidence in a systematic review 186 . In an RCT investigating a potential association between metformin and NAFLD, no improvement in NAFLD histology was found among people using metformin compared with those given placebo 187 . An RCT reported benefits of treatment with the glucagon-like peptide 1 receptor agonist dulaglutide on cognitive function in a post hoc analysis 188 , suggesting that trials designed specifically to test the effects of dulaglutide on cognitive function should be undertaken.
Glucose control
Another important consideration is glycaemic control, which appears to have variable effects on emerging complications. A meta-analysis found no association of glycaemic control with cancer risk among those with diabetes mellitus 189 , and an RCT found no effect of intensive glucose lowering on cognitive function in people with T2DM 190 . However, glycaemic control has been associated with improved physical function 191 , decreased COVID-19 mortality 192 and a decreased risk of NAFLD 193 in observational studies of patients with diabetes mellitus; notably, no RCTs have yet confirmed these associations.
Conclusions
With advances in the management of diabetes mellitus and associated increased life expectancy, the face of diabetes mellitus complications is changing. As the management of glycaemia and traditional complications of diabetes mellitus is optimized, we are beginning instead to see deleterious effects of diabetes mellitus on the liver, brain and other organs. Given the substantial burden and risk of these emerging complications, future clinical and public health strategies should be updated accordingly. There is a need to increase the awareness of emerging complications among primary care physicians at the frontline of diabetes mellitus care, and a place for screening for conditions such as depression, liver disease and cancers in diabetes mellitus guidelines should be considered. Clinical care for older people with diabetes mellitus should target physical activity, particularly strength-based activity, to reduce the risk of functional disability in ageing populations. Ongoing high-quality surveillance of diabetes mellitus outcomes is imperative to ensure we know where the main burdens lie. Given the growing burden of these emerging complications, the traditional management of diabetes mellitus might need to broaden its horizons.
Zimmet, P., Alberti, K. G. M. M. & Shaw, J. Global and societal implications of the diabetes epidemic. Nature 414 , 782–787 (2001).
Article CAS PubMed Google Scholar
Fowler, M. J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 26 , 77–82 (2008).
Article Google Scholar
Shah, A. D. et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol. 3 , 105–113 (2015).
Article PubMed PubMed Central Google Scholar
Bertoni, A. G. et al. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 27 , 699–703 (2004).
Article PubMed Google Scholar
Gregg, E. W. et al. Changes in diabetes-related complications in the United States, 1990–2010. N. Engl. J. Med. 370 , 1514–1523 (2014).
Gregg, E. W., Sattar, N. & Ali, M. K. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 4 , 537–547 (2016).
Gregg, E. W. et al. Trends in cause-specific mortality among adults with and without diagnosed diabetes in the USA: an epidemiological analysis of linked national survey and vital statistics data. Lancet 391 , 2430–2440 (2018).
Harding, J. L., Shaw, J. E., Peeters, A., Davidson, S. & Magliano, D. J. Age-specific trends from 2000–2011 in all-cause and cause-specific mortality in type 1 and type 2 diabetes: a cohort study of more than one million people. Diabetes Care 39 , 1018–1026 (2016).
Pearson-Stuttard, J. et al. Trends in predominant causes of death in individuals with and without diabetes in England from 2001 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 9 , 165–173 (2021).
Einarson, T. R., Acs, A., Ludwig, C. & Panton, U. H. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 17 , 83 (2018).
Pearson-Stuttard, J., Buckley, J., Cicek, M. & Gregg, E. W. The changing nature of mortality and morbidity in patients with diabetes. Endocrinol. Metab. Clin. North Am. 50 , 357–368 (2021).
Pearson-Stuttard, J. et al. Trends in leading causes of hospitalisation of adults with diabetes in England from 2003 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 10 , 46–57 (2022).
Pearson-Stuttard, J., Blundell, S., Harris, T., Cook, D. G. & Critchley, J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 4 , 148–158 (2016).
Tolman, K. G., Fonseca, V., Dalpiaz, A. & Tan, M. H. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care 30 , 734–743 (2007).
Chatterjee, S. et al. Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 39 , 300–307 (2016).
Tsilidis, K. K., Kasimis, J. C., Lopez, D. S., Ntzani, E. E. & Ioannidis, J. P. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 350 , g7607 (2015).
Harding, J. L., Pavkov, M. E., Magliano, D. J., Shaw, J. E. & Gregg, E. W. Global trends in diabetes complications: a review of current evidence. Diabetologia 62 , 3–16 (2019).
Unal, B., Critchley, J. A. & Capewell, S. Explaining the decline in coronary heart disease mortality in England and Wales between 1981 and 2000. Circulation 109 , 1101–1107 (2004).
Pearson-Stuttard, J., Ezzati, M. & Gregg, E. W. Multimorbidity — a defining challenge for health systems. Lancet Public. Health 4 , e599–e600 (2019).
Lee, L., Cheung, W. Y., Atkinson, E. & Krzyzanowska, M. K. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J. Clin. Oncol. 29 , 106–117 (2011).
Srokowski, T. P., Fang, S., Hortobagyi, G. N. & Giordano, S. H. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J. Clin. Oncol. 27 , 2170–2176 (2009).
Gross, C. P., McAvay, G. J., Guo, Z. & Tinetti, M. E. The impact of chronic illnesses on the use and effectiveness of adjuvant chemotherapy for colon cancer. Cancer 109 , 2410–2419 (2007).
Harding, J. L. et al. All-cause cancer mortality over 15 years in multi-ethnic Mauritius: the impact of diabetes and intermediate forms of glucose tolerance. Int. J. Cancer 131 , 2385–2393 (2012).
Wang, C. et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int. J. Cancer 130 , 1639–1648 (2012).
El-Serag, H. B., Hampel, H. & Javadi, F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin. Gastroenterol. Hepatol. 4 , 369–380 (2006).
Desbois, A. C. & Cacoub, P. Diabetes mellitus, insulin resistance and hepatitis C virus infection: a contemporary review. World J. Gastroenterol. 23 , 1697–1711 (2017).
Article CAS PubMed PubMed Central Google Scholar
Huxley, R., Ansary-Moghaddam, A., Berrington De González, A., Barzi, F. & Woodward, M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br. J. Cancer 92 , 2076–2083 (2005).
Gullo, L. et al. Diabetes and the risk of pancreatic cancer. N. Engl. J. Med. 331 , 81–84 (1994).
Gershell, L. Type 2 diabetes market. Nat. Rev. Drug Discov. 4 , 367–368 (2005).
Carstensen, B. et al. Cancer incidence in persons with type 1 diabetes: a five-country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia 59 , 980–988 (2016).
Jiang, Y. et al. Diabetes mellitus and incidence and mortality of colorectal cancer: a systematic review and meta-analysis of cohort studies. Eur. J. Epidemiol. 26 , 863–876 (2011).
De Bruijn, K. M. J. et al. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br. J. Surg. 100 , 1421–1429 (2013).
Deng, L., Gui, Z., Zhao, L., Wang, J. & Shen, L. Diabetes mellitus and the incidence of colorectal cancer: an updated systematic review and meta-analysis. Dig. Dis. Sci. 57 , 1576–1585 (2012).
Liao, C., Zhang, D., Mungo, C., Andrew Tompkins, D. & Zeidan, A. M. Is diabetes mellitus associated with increased incidence and disease-specific mortality in endometrial cancer? A systematic review and meta-analysis of cohort studies. Gynecol. Oncol. 135 , 163–171 (2014).
Saed, L. et al. The effect of diabetes on the risk of endometrial cancer: an updated a systematic review and meta-analysis. BMC Cancer 19 , 527 (2019).
Friberg, E., Orsini, N., Mantzoros, C. S. & Wolk, A. Diabetes mellitus and risk of endometrial cancer: A meta-analysis. Diabetologia 50 , 1365–1374 (2007).
Anothaisintawee, T. et al. Risk factors of breast cancer: a systematic review and meta-analysis. Asia-Pac. J. Public Health 25 , 368–387 (2013).
Larsson, S. C., Mantzoros, C. S. & Wolk, A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int. J. Cancer 121 , 856–862 (2007).
Boyle, P. et al. Diabetes and breast cancer risk: a meta-analysis. Br. J. Cancer 107 , 1608–1617 (2012).
Rinaldi, S. et al. Anthropometric measures, endogenous sex steroids and breast cancer risk in postmenopausal women: a study within the EPIC cohort. Int. J. Cancer 118 , 2832–2839 (2006).
Michels, K. B. et al. Type 2 diabetes and subsequent incidence of breast cancer in the nurses’ health study. Diabetes Care 26 , 1752–1758 (2003).
Bronsveld, H. K. et al. Diabetes and breast cancer subtypes. PLoS ONE 12 , e0170084 (2017).
Article PubMed PubMed Central CAS Google Scholar
Zhang, D., Li, N., Xi, Y., Zhao, Y. & Wang, T. Diabetes mellitus and risk of ovarian cancer. A systematic review and meta-analysis of 15 cohort studies. Diabetes Res. Clin. Pract. 130 , 43–52 (2017).
Weng, L., Wang, L., Zhang, J., Wang, B. & Liu, H. Association between diabetes mellitus and subsequent ovarian cancer in women: a systematic review and meta-analysis of cohort studies. Medicine 96 , e6396 (2017).
Wang, L., Zhong, L., Xu, B., Chen, M. & Huang, H. Diabetes mellitus and the risk of ovarian cancer: a systematic review and meta-analysis of cohort and case-control studies. BMJ Open 10 , e040137 (2020).
Lee, J. Y. et al. Diabetes mellitus and ovarian cancer risk: a systematic review and meta-analysis of observational studies. Int. J. Gynecol. Cancer 23 , 402–412 (2013).
Bonovas, S., Filioussi, K. & Tsantes, A. Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia 47 , 1071–1078 (2004).
Shikata, K., Ninomiya, T. & Kiyohara, Y. Diabetes mellitus and cancer risk: review of the epidemiological evidence. Cancer Sci. 104 , 9–14 (2013).
Long, X. J., Lin, S., Sun, Y. N. & Zheng, Z. F. Diabetes mellitus and prostate cancer risk in Asian countries: a meta-analysis. Asian Pac. J. Cancer Preven. 13 , 4097–4100 (2012).
Rhee, E. J. Diabetes in Asians. Endocrinol. Metab. 30 , 263–269 (2015).
Article CAS Google Scholar
Bensimon, L., Yin, H., Suissa, S., Pollak, M. N. & Azoulay, L. Type 2 diabetes and the risk of mortality among patients with prostate cancer. Cancer Causes Control. 25 , 329–338 (2014).
Johnson, J. A., Bowker, S. L., Richardson, K. & Marra, C. A. Time-varying incidence of cancer after the onset of type 2 diabetes: evidence of potential detection bias. Diabetologia 54 , 2263–2271 (2011).
Johnson, J. A. et al. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia 55 , 1607–1618 (2012).
Harding, J. L., Shaw, J. E., Peeters, A., Cartensen, B. & Magliano, D. J. Cancer risk among people with type 1 and type 2 diabetes: disentangling true associations, detection bias, and reverse causation. Diabetes Care 38 , 264–270 (2015).
Pearson-Stuttard, J. et al. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol. 6 , e6–e15 (2018).
Larsson, S. C. & Wolk, A. Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia 54 , 1013–1018 (2011).
Xu, X. et al. Diabetes mellitus and risk of bladder cancer: a meta-analysis of cohort studies. PLoS ONE 8 , e58079 (2013).
Gong, I. Y. et al. Association between diabetes and haematological malignancies: a population-based study. Diabetologia 64 , 540–551 (2021).
Giovannucci, E. et al. Diabetes and cancer: a consensus report. Diabetes Care 33 , 1674–1685 (2010).
Weinstein, D., Simon, M., Yehezkel, E., Laron, Z. & Werner, H. Insulin analogues display IGF-I-like mitogenic and anti-apoptotic activities in cultured cancer cells. Diabetes Metab. Res. Rev. 25 , 41–49 (2009).
Najjar, S. M. & Perdomo, G. Hepatic insulin clearance: mechanism and physiology. Physiology 34 , 198–215 (2019).
Lorenzi, M., Montisano, D. F., Toledo, S. & Barrieux, A. High glucose induces DNA damage in cultured human endothelial cells. J. Clin. Invest. 77 , 322–325 (1986).
Robertson, R., Zhou, H., Zhang, T. & Harmon, J. S. Chronic oxidative stress as a mechanism for glucose toxicity of the beta cell in type 2 diabetes. Cell Biochem. Biophys. 48 , 139–146 (2007).
Turturro, F., Friday, E. & Welbourne, T. Hyperglycemia regulates thioredoxin-ROS activity through induction of thioredoxin-interacting protein (TXNIP) in metastatic breast cancer-derived cells MDA-MB-231. BMC Cancer 7 , 96 (2007).
Wu, Y., Liu, Y., Dong, Y. & Vadgama, J. Diabetes-associated dysregulated cytokines and cancer. Integr. Cancer Sci. Ther. 3 , 370–378 (2016).
PubMed PubMed Central Google Scholar
Inoki, K., Kim, J. & Guan, K. L. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu. Rev. Pharmacol. Toxicol. 52 , 381–400 (2012).
Huang, X., Liu, G., Guo, J. & Su, Z. Q. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 14 , 1483–1496 (2018).
Zhao, Y. et al. Metformin is associated with reduced cell proliferation in human endometrial cancer by inbibiting PI3K/AKT/mTOR signaling. Gynecol. Endocrinol. 34 , 428–432 (2018).
Knapp, S. Diabetes and infection: is there a link?-A mini-review. Gerontology 59 , 99–104 (2013).
Fang, M. et al. Diabetes and the risk of hospitalisation for infection: the atherosclerosis risk in communities (ARIC) study. Diabetologia 64 , 2458–2465 (2021).
Tseng, C.-H. Metformin use is associated with a reduced risk of acute appendicitis in Taiwanese patients with type 2 diabetes mellitus. Sci. Rep. 11 , 12400 (2021).
Luk, A. O. Y. et al. Temporal trends in rates of infection-related hospitalisations in Hong Kong people with and without diabetes, 2001–2016: a retrospective study. Diabetologia 64 , 109–118 (2021).
Magliano, D. J. et al. Excess risk of dying from infectious causes in those with type 1 and type 2 diabetes. Diabetes Care 38 , 1274–1280 (2015).
Martin, E. T. et al. Diabetes and risk of surgical site infection: a systematic review and meta-analysis. Infect. Control. Hosp. Epidemiol. 37 , 88–99 (2016).
Trussell, J. et al. Impact of a patient care pathway protocol on surgical site infection rates in cardiothoracic surgery patients. Am. J. Surg. 196 , 883–889 (2008).
Coleman, J. S. et al. Surgical site infections after hysterectomy among HIV-infected women in the HAART era: a single institution’s experience from 1999–2012. Am. J. Obstet. Gynecol. 210 , 117.e111–117.e117 (2014).
Friedman, N. D., Sexton, D. J., Connelly, S. M. & Kaye, K. S. Risk factors for surgical site infection complicating laminectomy. Infect. Control. Hosp. Epidemiol. 28 , 1060–1065 (2007).
Apicella, M. et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 8 , 782–792 (2020).
McGurnaghan, S. J. et al. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 9 , 82–93 (2021).
Rawshani, A. et al. Severe COVID-19 in people with type 1 and type 2 diabetes in Sweden: a nationwide retrospective cohort study. Lancet Reg. Health Eur. 4 , 100105 (2021).
You, J. H. et al. Clinical outcomes of COVID-19 patients with type 2 diabetes: a population-based study in Korea. Endocrinol. Metab. 35 , 901–908 (2020).
Moon, S. J. et al. Independent impact of diabetes on the severity of coronavirus disease 2019 in 5,307 patients in South Korea: a nationwide cohort study. Diabetes Metab. J. 44 , 737–746 (2020).
Aranjani, J. M., Manuel, A., Razack, H. I. A. & Mathew, S. T. Covid-19–associated mucormycosis: evidence-based critical review of an emerging infection burden during the pandemic’s second wave in India. PLoS. Negl. Trop. Dis. 15 , e0009921 (2021).
Crankson, S., Pokhrel, S. & Anokye, N. K. Determinants of COVID-19-related length of hospital stays and long COVID in Ghana: a cross-sectional analysis. Int. J. Environ. Res. Public Health 19 , 527 (2022).
Bellan, M. et al. Respiratory and psychophysical sequelae among patients with covid-19 four months after hospital discharge. JAMA Netw. Open 4 , e2036142 (2021).
Gottesman, B. L., Yu, J., Tanaka, C., Longhurst, C. A. & Kim, J. J. Incidence of new-onset type 1 diabetes among US children during the COVID-19 global pandemic. JAMA Pediatr. 176 , 414–415 (2022).
Barrett, C. E. et al. Risk for newly diagnosed diabetes <30 days after SARS-CoV-2 infection among persons aged >18 years-United States, March 1, 2020-June 28, 2021. Morb. Mortal. Wkly. Rep. 71 , 59–65 (2022).
Kornum, J. B. et al. Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care 31 , 1541–1545 (2008).
Matsuyama, R., Nishiura, H., Kutsuna, S., Hayakawa, K. & Ohmagari, N. Clinical determinants of the severity of Middle East respiratory syndrome (MERS): a systematic review and meta-analysis. BMC Public Health 16 , 1203 (2016).
Badawi, A. & Ryoo, S. G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int. J. Infect. Dis. 49 , 129–133 (2016).
Badawi, A. & Ryoo, S. G. Prevalence of diabetes in the 2009 influenza A (H1N1) and the middle east respiratory syndrome coronavirus: a systematic review and meta-analysis. J. Public. Health Res. 5 , 130–138 (2016).
Yang, J. K. et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. 23 , 623–628 (2006).
Ehrlich, S. F., Quesenberry, C. P. Jr, Van Den Eeden, S. K., Shan, J. & Ferrara, A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care 33 , 55–60 (2010).
Alraddadi, B. M. et al. Risk factors for primary middle east respiratory syndrome coronavirus illness in humans, Saudi Arabia, 2014. Emerg. Infect. Dis. 22 , 49–55 (2016).
Geerlings, S. E. & Hoepelman, A. I. M. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 26 , 259–265 (1999).
Velazquez-Salinas, L., Verdugo-Rodriguez, A., Rodriguez, L. L. & Borca, M. V. The role of interleukin 6 during viral infections. Front. Microbiol. 10 , 1057 (2019).
Joshi, N., Caputo, G. M., Weitekamp, M. R. & Karchmer, A. W. Infections in patients with diabetes mellitus. N. Engl. J. Med. 341 , 1906–1912 (1999).
Codo, A. C. et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/Glycolysis-dependent axis. Cell Metab. 32 , 437–446.e435 (2020).
Miyazawa, T., Nakagawa, K., Shimasaki, S. & Nagai, R. Lipid glycation and protein glycation in diabetes and atherosclerosis. Amino Acids 42 , 1163–1170 (2012).
Peleg, A. Y., Weerarathna, T., McCarthy, J. S. & Davis, T. M. E. Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes Metab. Res. Rev. 23 , 3–13 (2007).
Barda, N., Dagan, N. & Balicer, R. D. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. reply. N. Engl. J. Med. 384 , 1970 (2021).
PubMed Google Scholar
Cholankeril, G. & Ahmed, A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the United States. Clin. Gastroenterol. Hepatol. 16 , 1356–1358 (2018).
Fink, M. & Byrne, M. Australia and New Zealand Liver and Intestinal Transplant Registry Annual Report 2019 (Melbourne, Victoria, Australia, 2019).
Haldar, D. et al. Outcomes of liver transplantation for non-alcoholic steatohepatitis: a European liver transplant registry study. J. Hepatol. 71 , 313–322 (2019).
Younossi, Z. M. et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J. Hepatol. 71 , 793–801 (2019).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64 , 73–84 (2016).
Pang, Y. et al. Diabetes, plasma glucose, and incidence of fatty liver, cirrhosis, and liver cancer: a prospective study of 0.5 million people. Hepatology 68 , 1308–1318 (2018).
Li, Y. et al. Bidirectional association between nonalcoholic fatty liver disease and type 2 diabetes in Chinese population: evidence from the Dongfeng-Tongji cohort study. PLoS ONE 12 , e0174291 (2017).
Mansour-Ghanaei, F. et al. Prevalence of non-alcoholic fatty liver disease in patients with diabetes mellitus, hyperlipidemia, obesity and polycystic ovary syndrome: a cross-sectional study in north of Iran. Diabetes Metab. Syndr. 13 , 1591–1596 (2019).
Leite, N. C., Salles, G. F., Araujo, A. L., Villela-Nogueira, C. A. & Cardoso, C. R. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 29 , 113–119 (2009).
Singh, S. P. et al. Risk factors associated with non-alcoholic fatty liver disease in Indians: a case-control study. J. Clin. Exp. Hepatol. 5 , 295–302 (2015).
Dufour, J.-F. et al. The global epidemiology of nonalcoholic steatohepatitis (NASH) and associated risk factors–a targeted literature review. Endocr. Metab. Sci. 3 , 100089 (2021).
Loomba, R. et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 56 , 943–951 (2012).
Anstee, Q. M., Targher, G. & Day, C. P. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 10 , 330–344 (2013).
Holstein, A., Hinze, S., Thießen, E., Plaschke, A. & Egberts, E. H. Clinical implications of hepatogenous diabetes in liver cirrhosis. J. Gastroenterol. Hepatol. 17 , 677–681 (2002).
Del Vecchio Blanco, C., Gentile, S., Marmo, R., Carbone, L. & Coltorti, M. Alterations of glucose metabolism in chronic liver disease. Diabetes Res. Clin. Pract. 8 , 29–36 (1990).
Zein, N. N., Abdulkarim, A. S., Wiesner, R. H., Egan, K. S. & Persing, D. H. Prevalence of diabetes mellitus in patients with end-stage liver cirrhosis due to hepatitis C, alcohol, or cholestatic disease. J. Hepatol. 32 , 209–217 (2000).
Niederau, C. et al. Survival and causes of death in cirrhotic and in noncirrhotic patients with primary hemochromatosis. N. Engl. J. Med. 313 , 1256–1262 (1985).
Larter, C. Z. & Farrell, G. C. Insulin resistance, adiponectin, cytokines in NASH: which is the best target to treat? J. Hepatol. 44 , 253–261 (2006).
Marchesini, G. et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am. J. Med. 107 , 450–455 (1999).
Angulo, P. Medical progress: nonalcoholic fatty liver disease. N. Engl. J. Med. 346 , 1221–1231 (2002).
Porepa, L., Ray, J. G., Sanchez-Romeu, P. & Booth, G. L. Newly diagnosed diabetes mellitus as a risk factor for serious liver disease. CMAJ 182 , E526–E531 (2010).
Stefan, N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 8 , 616–627 (2020).
Stefan, N., Häring, H. U. & Cusi, K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 7 , 313–324 (2019).
Sattar, N. & Gill, J. M. R. Type 2 diabetes as a disease of ectopic fat? BMC Med. 12 , 123 (2014).
Harding, K. A. et al. Depression prevalence in type 2 diabetes is not related to diabetes–depression symptom overlap but is related to symptom dimensions within patient self-report measures: a meta-analysis. Diabet. Med. 36 , 1600–1611 (2019).
Lim, G. Y. et al. Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci. Rep. 8 , 2861 (2018).
Roy, T. & Lloyd, C. E. Epidemiology of depression and diabetes: a systematic review. J. Affect. Disord. 142 , S8–S21 (2012).
Rotella, F. & Mannucci, E. Diabetes mellitus as a risk factor for depression. A meta-analysis of longitudinal studies. Diabetes Res. Clin. Pract. 99 , 98–104 (2013).
Nouwen, A. et al. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia 53 , 2480–2486 (2010).
Grigsby, A. B., Anderson, R. J., Freedland, K. E., Clouse, R. E. & Lustman, P. J. Prevalence of anxiety in adults with diabetes a systematic review. J. Psychosom. Res. 53 , 1053–1060 (2002).
Smith, K. J. et al. Association of diabetes with anxiety: a systematic review and meta-analysis. J. Psychosom. Res. 74 , 89–99 (2013).
Young, V. et al. Eating problems in adolescents with type1 diabetes: a systematic review with meta-analysis. Diabet. Med. 30 , 189–198 (2013).
Schabert, J., Browne, J. L., Mosely, K. & Speight, J. Social stigma in diabetes: a framework to understand a growing problem for an increasing epidemic. Patient 6 , 1–10 (2013).
Barnard, K. D., Speight, J. & Skinner, T. C. Quality of life and impact of continuous subcutaneous insulin infusion for children and their parents. Pract. Diabetes Int. 25 , 278–283 (2008).
Hagger, V., Hendrieckx, C., Sturt, J., Skinner, T. C. & Speight, J. Diabetes distress among adolescents with type 1 diabetes: a systematic review. Curr. Diabetes Rep. 16 , 1–14 (2016).
Abdoli, S. et al. New insights into diabetes burnout and its distinction from diabetes distress and depressive symptoms: a qualitative study. Diabetes Res. Clin. Pract. 169 , 108446 (2020).
Pickup, J. C. & Crook, M. A. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia 41 , 1241–1248 (1998).
Dantzer, R., O’Connor, J. C., Freund, G. G., Johnson, R. W. & Kelley, K. W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9 , 46–56 (2008).
Prestele, S., Aldenhoff, J. & Reiff, J. [The HPA-axis as a possible link between depression, diabetes mellitus and cognitive dysfunction]. Fortschr. Neurol. Psychiatr. 71 , 24–36 (2003).
Cole, J., Costafreda, S. G., McGuffin, P. & Fu, C. H. Hippocampal atrophy in first episode depression: a meta-analysis of magnetic resonance imaging studies. J. Affect. Disord. 134 , 483–487 (2011).
Gold, S. M. et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 50 , 711–719 (2007).
Moulton, C. D., Costafreda, S. G., Horton, P., Ismail, K. & Fu, C. H. Y. Meta-analyses of structural regional cerebral effects in type 1 and type 2 diabetes. Brain Imaging Behav. 9 , 651–662 (2015).
Khalil, M., Power, N., Graham, E., Deschênes, S. S. & Schmitz, N. The association between sleep and diabetes outcomes – systematic review. Diabetes Res. Clin. Pract. 161 , 108035 (2020).
Senaratna, C. V. et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep. Med. Rev. 34 , 70–81 (2017).
Subramanian, A. et al. Risk of incident obstructive sleep apnea among patients with type 2 diabetes. Diabetes Care 42 , 954–963 (2019).
Huang, T. et al. A population-based study of the bidirectional association between obstructive sleep apnea and type 2 diabetes in three prospective U.S. Cohorts. Diabetes Care 41 , 2111–2119 (2018).
Reutrakul, S. et al. Sleep characteristics in type 1 diabetes and associations with glycemic control: systematic review and meta-analysis. Sleep. Med. 23 , 26–45 (2016).
Nagayoshi, M. et al. Obstructive sleep apnea and incident type 2 diabetes. Sleep. Med. 25 , 156–161 (2016).
Ficker, J. H. et al. Obstructive sleep apnoea and diabetes mellitus: the role of cardiovascular autonomic neuropathy. Eur. Respir. J. 11 , 14–19 (1998).
Young, T., Peppard, P. E. & Taheri, S. Excess weight and sleep-disordered breathing. J. Appl. Physiol. 99 , 1592–1599 (2005).
Ip, M. S. M. et al. Obstructive sleep apnea is independently associated with insulin resistance. Am. J. Respir. Crit. Care Med. 165 , 670–676 (2002).
Shaw, J. E. et al. The effect of treatment of obstructive sleep apnea on glycemic control in type 2 diabetes. Am. J. Respir. Crit. Care Med. 194 , 486–492 (2016).
Lu, F. P., Lin, K. P. & Kuo, H. K. Diabetes and the risk of multi-system aging phenotypes: A systematic review and meta-analysis. PLoS ONE 4 , e4144 (2009).
Cheng, G., Huang, C., Deng, H. & Wang, H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern. Med. J. 42 , 484–491 (2012).
Li, X. Y. et al. Midlife modifiable risk factors for dementia: a systematic review and meta-analysis of 34 prospective cohort studies. Curr. Alzheimer Res. 16 , 1254–1268 (2019).
Article PubMed CAS Google Scholar
Xue, M. et al. Diabetes mellitus and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 144 prospective studies. Ageing Res. Rev. 55 , 100944 (2019).
Pal, K., Mukadam, N., Petersen, I. & Cooper, C. Mild cognitive impairment and progression to dementia in people with diabetes, prediabetes and metabolic syndrome: a systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 53 , 1149–1160 (2018).
Biessels, G. J., Staekenborg, S., Brunner, E., Brayne, C. & Scheltens, P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 5 , 64–74 (2006).
Peila, R., Rodriguez, B. L. & Launer, L. J. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia aging study. Diabetes 51 , 1256–1262 (2002).
Abner, E. L. et al. Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimer’s Dement. 12 , 882–889 (2016).
Matioli, M. N. P. S. et al. Association between diabetes and causes of dementia: evidence from a clinicopathological study. Dement. Neuropsychol. 11 , 406–412 (2017).
You, Y. et al. The prevalence of mild cognitive impairment in type 2 diabetes mellitus patients: a systematic review and meta-analysis. Acta Diabetol. 58 , 671–685 (2021).
Langa, K. M. & Levine, D. A. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA 312 , 2551–2561 (2014).
Pelimanni, E. & Jehkonen, M. Type 2 diabetes and cognitive functions in middle age: a meta-analysis. J. Int. Neuropsychol. Soc. 25 , 215–229 (2019).
Rom, S. et al. Hyperglycemia and advanced glycation end products disrupt BBB and promote occludin and claudin-5 protein secretion on extracellular microvesicles. Sci. Rep. 10 , 7274 (2020).
Hussain, B., Fang, C. & Chang, J. Blood–brain barrier breakdown: an emerging biomarker of cognitive impairment in normal aging and dementia. Front. Neurosci. 15 , 688090 (2021).
Anstey, K. J., Sargent-Cox, K., Eramudugolla, R., Magliano, D. J. & Shaw, J. E. Association of cognitive function with glucose tolerance and trajectories of glucose tolerance over 12 years in the AusDiab study. Alzheimers Res. Ther. 7 , 48 (2015).
Steen, E. et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease - Is this type 3 diabetes? J. Alzheimer’s Dis. 7 , 63–80 (2005).
Leonardi, M., Bickenbach, J., Ustun, T. B., Kostanjsek, N. & Chatterji, S. The definition of disability: what is in a name? Lancet 368 , 1219–1221 (2006).
Lisy, K., Campbell, J. M., Tufanaru, C., Moola, S. & Lockwood, C. The prevalence of disability among people with cancer, cardiovascular disease, chronic respiratory disease and/or diabetes: a systematic review. Int. J. Evid. Based Healthc. 16 , 154–166 (2018).
Yang, Y., Hu, X., Zhang, Q. & Zou, R. Diabetes mellitus and risk of falls in older adults: a systematic review and meta-analysis. Age Ageing 45 , 761–767 (2016).
Wong, E. et al. Diabetes and risk of physical disability in adults: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 1 , 106–114 (2013).
Havercamp, S. M., Scandlin, D. & Roth, M. Health disparities among adults with developmental disabilities, adults with other disabilities, and adults not reporting disability in North Carolina. Public. Health Rep. 119 , 418–426 (2004).
Herquelot, E., Guéguen, A., Bonenfant, S. & Dray-Spira, R. Impact of diabetes on work cessation: data from the GAZEL cohort study. Diabetes Care 34 , 1344–1349 (2011).
Virtanen, M. et al. Work disability among employees with diabetes: latent class analysis of risk factors in three prospective cohort studies. PLoS ONE 10 , e0143184 (2015).
Cho, N. H. et al. IDF Diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 138 , 271–281 (2018).
Seok, W. P. et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care 30 , 1507–1512 (2007).
Stratton, I. M. et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br. Med. J. 321 , 405–412 (2000).
DeCensi, A. et al. Metformin and cancer risk in diabetic patients: A systematic review and meta-analysis. Cancer Prev. Res. 3 , 1451–1461 (2010).
Inzucchi, S. E., Lipska, K. J., Mayo, H., Bailey, C. J. & McGuire, D. K. Metformin in patientswith type 2 diabetes and kidney disease a systematic review. JAMA 312 , 2668–2675 (2014).
Suissa, S. & Azoulay, L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 35 , 2665–2673 (2012).
Karlstad, Ø. et al. Use of insulin and insulin analogs and risk of cancer-systematic review and meta-analysis of observational studies. Curr. Drug. Saf. 8 , 333–348 (2013).
Bordeleau, L. et al. The association of basal insulin glargine and/or n-3 fatty acids with incident cancers in patients with dysglycemia. Diabetes Care 37 , 1360–1366 (2014).
Guo, M. et al. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin. Exp. Pharmacol. Physiol. 41 , 650–656 (2014).
CAS PubMed Google Scholar
Campbell, J. M. et al. Metformin use associated with reduced risk of dementia in patients with diabetes: a systematic review and meta-analysis. J. Alzheimer’s Dis. 65 , 1225–1236 (2018).
Haukeland, J. W. et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand. J. Gastroenterol. 44 , 853–860 (2009).
Cukierman-Yaffe, T. et al. Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol. 19 , 582–590 (2020).
Johnson, J. A. & Bowker, S. L. Intensive glycaemic control and cancer risk in type 2 diabetes: a meta-analysis of major trials. Diabetologia 54 , 25–31 (2011).
Launer, L. J. et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. Lancet Neurol. 10 , 969–977 (2011).
Jia, Y. et al. Associations of the glycaemic control of diabetes with dementia and physical function in rural-dwelling older Chinese adults: a population-based study. Clin. Interv. Aging 16 , 1503–1513 (2021).
Lesniak, C. et al. Inpatient glycemic control and outcome of COVID-19 patients: a retrospective cohort. SAGE Open. Med. 9 , 20503121211039105 (2021).
Afolabi, B. I. et al. The relationship between glycaemic control and non-alcoholic fatty liver disease in Nigerian type 2 diabetic patients. J. Natl Med. Assoc. 110 , 256–264 (2018).
Nouwen, A. et al. Longitudinal associations between depression and diabetes complications: a systematic review and meta-analysis. Diabet. Med. 36 , 1562–1572 (2019).
Perry, B. D. et al. Muscle atrophy in patients with Type 2 diabetes mellitus: roles of inflammatory pathways, physical activity and exercise. Exerc. Immunol. Rev. 22 , 94–109 (2016).
Hirata, Y. et al. Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. JCI Insight 4 , e124952 (2019).
Article PubMed Central Google Scholar
Bassil, M. S. & Gougeon, R. Muscle protein anabolism in type 2 diabetes. Curr. Opin. Clin. Nutr. Metab. Care 16 , 83–88 (2013).
Meex, R. C. R., Blaak, E. E. & van Loon, L. J. C. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes. Rev. 20 , 1205–1217 (2019).
Download references
Acknowledgements
D.T. is supported by an Australian Government Research Training Program (RTP) Scholarship and Monash Graduate Excellence Scholarship. J.E.S. is supported by a National Health and Medical Research Council Investigator Grant. D.J.M. is supported by a National Health and Medical Research Council Senior Research Fellowship.
Author information
These authors jointly supervised this work: Jonathan E. Shaw and Dianna J. Magliano.
Authors and Affiliations
Baker Heart and Diabetes Institute, Melbourne, Victoria, Australia
Dunya Tomic, Jonathan E. Shaw & Dianna J. Magliano
School of Public Health and Preventive Medicine, Monash University, Melbourne, Victoria, Australia
You can also search for this author in PubMed Google Scholar
Contributions
D.T. researched data for the article and wrote the article. J.E.S and D.J.M. contributed substantially to discussion of the content. D.T., J.E.S. and D.J.M reviewed and/or edited the manuscript before submission.
Corresponding author
Correspondence to Dianna J. Magliano .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Endocrinology thanks Emily Gallagher, Norbert Stefan and Assaad Eid for their contribution to the peer review of this work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fundamental skills required to independently care for oneself such as eating, bathing and mobility.
Activities that allow an individual to live independently in a community.
The error in estimating the association between an exposure and an outcome that results from misclassification or exclusion of time intervals.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Tomic, D., Shaw, J.E. & Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol 18 , 525–539 (2022). https://doi.org/10.1038/s41574-022-00690-7
Download citation
Accepted : 06 May 2022
Published : 06 June 2022
Issue Date : September 2022
DOI : https://doi.org/10.1038/s41574-022-00690-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Unhealthy plant-based diet is associated with a higher cardiovascular disease risk in patients with prediabetes and diabetes: a large-scale population-based study.
- Fenglei Wang
- Jingjing Jiao
BMC Medicine (2024)
Hyperglycemia enhances brain susceptibility to lipopolysaccharide-induced neuroinflammation via astrocyte reprogramming
- Kyung-Seo Lee
- Sung-Hyun Yoon
Journal of Neuroinflammation (2024)
Application of the path analysis model to evaluate the role of distress, mental health literacy and burnout in predicting self-care behaviors among patients with type 2 diabetes
- Alireza Jafari
- Mahdi Moshki
- Hassan Alizadeh
Diabetology & Metabolic Syndrome (2024)
Epigenetic mechanisms in cardiovascular complications of diabetes: towards future therapies
- Giulia Damiano
- Raffaella Rinaldi
- Maria Cristina Vinci
Molecular Medicine (2024)
Gender-specific accuracy of lipid accumulation product index for the screening of metabolic syndrome in general adults: a meta-analysis and comparative analysis with other adiposity indicators
- Bendix Samarta Witarto
- Andro Pramana Witarto
- Delvac Oceandy
Lipids in Health and Disease (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

IMAGES
VIDEO