123 Alzheimer’s Disease Essay Topics & Examples
If you’re writing about patients with memory loss or dementia care and treatment, this article will be of use. Our team has prepared Alzheimer’s disease essay examples and topics below.

🏆 Best Alzheimer’s Disease Essay Examples & Topics
💡 most interesting alzheimer’s disease topics to write about, 📌 simple & easy alzheimer’s disease research topics, 👍 good research topics about alzheimer’s disease, ❓ research questions about alzheimer’s disease.
- Early Diagnosis and Imaging Techniques for Alzheimer’s Disease: MRI and PET Scans This paper aims to cover the Early Identification of Patients in the Pre-Demented Stage of Alzheimer’s Disease and methods that can be used to detect it.
- Alzheimer’s Disease: The Stem Cell Therapy The task of disposing of unused frozen human embryos differs from disposing of other medical tissues. Similarly, before disposing of the embryo, other individuals might need to perform cultural traditions with or for it.
- Physician-Assisted Suicide, Alzheimer’s Disease, and Genetics In other words, it is the study of genes and traits passed from parents to offspring and the differences in traits, such as physical and mental traits.
- Alzheimer’s Disease: Making Decisions for Patient Based on the condition of the patient, the best decision to manage the condition is through the use of cognitive behavioral strategies.
- Alzheimer’s Disease: Debilitating Neurological Ailment A sound knowledge of Alzheimer’s disease should give a better comprehension of the aging process, insights into how brain function changes in individuals with Alzheimer’s disease, and a viewpoint on how to delay or prevent […]
- The Study of Alzheimer’s Disease Therefore, the study of Alzheimer’s disease will help to gain an understanding of its characteristics, consequences, and methods of treatment among the older generation.
- The Case Study of Patient With Late-Stage Alzheimer’s Disease In the majority of cases of Alzheimer’s, it has been shown that patients are unable to make decisions on their own and are also unable to communicate their assent verbally.
- Therapeutic Dogs, Dementia, Alzheimer’s and Fluid Intelligence It is worth noting that with dementia, the patient has a speech disorder and a personality change in the early stages of the pathology.
- The Alzheimer’s Association Dementia Care Practice Therefore, achieving the philosophy and recommendations of the association is a shared responsibility between doctors, patients, and caregivers. Ultimately, CAPD tests the functionalities of the patient ranging from the psychomotor activities, perceptions, awareness, and orientations, […]
- Dementia, Alzheimer, and Delirium in an Elderly Woman Additionally, she struggles with identifying the appropriate words to use in dialogue and changes the topic. Timing: While in the middle of conversations and public places like supermarkets.
- Alzheimer’s Disease Diagnosis and Intervention The accumulation of plaques and tangles in the brain is a hallmark of the disease, resulting in the death of neurons and a decline in mental capacity.
- Alzheimer’s Disease: Assessment and Intervention The caregiver is recommended to install safety locks and alarms on all doors and windows to prevent the patient from leaving the apartment without supervision.
- Management of a Patient With Alzheimer’s: Case Study The correlation between this issue and the probability of the emergence of AD in elderly citizens is proved by the scholars who examined the impact of the quality of air on a person’s health.
- Bilinguals’ Cognitive-Linguistic Abilities and Alzheimer’s Disease This irregularity is reflected in the preserved linguistic abilities, including code-switching and semantic fluency, and the declined functions in translation, picture naming, and phonemic fluency, calling for improved therapy and testing practices.
- Managing Dementia and Alzheimer’s Disease The PICOT question is “In the care of Alzheimer’s and dementia patients, does integrated community-based care as compared to being in a long-term care facility improve outcome throughout the remainder of their lives”.
- Pathophysiology of Alzheimer’s Disease The study will discuss the pathophysiology of Alzheimer’s disease, such as risk factors, cellular involvement, genetic influences, and the interventions of the available therapy’s pharmacological Interventions.
- Alzheimer’s Disease: Definition, Stages, Diagnosis Alzheimer’s disease is the most common type of dementia, and it is a condition in which the brain stops appropriately performing its functions.
- Fall Risk Assessment of Alzheimer’s Patient The nurse answers questions about the old lady helps fill the Stay Independent brochure and assists the observing physician in carrying the various clinical tests on the patient.
- Alzheimer’s Disease in an Iranian Patient The patient in the company of his son returns to the clinic after four weeks. Since the patient shows no side effects of the disease and an increase in Exelon to 6 mg orally BID […]
- Mr. Akkad and Alzheimer’s Disease: Case Study The onset of the symptoms is reported to have been within the past two years, but the situation has begun to deteriorate, prompting Mr.
- Alzheimer’s Disease: History, Mechanisms and Treatment Nevertheless, researchers state that the development of Alzheimer’s is impacted by the formation of protein plaques and tangles in the brain.
- Alzheimer’s Disease: Causes and Treatment AD is associated with different changes, both cognitive and behavioral. A patient can observe some or all of them depending on the development of the disease.
- Frontotemporal Dementia vs. Alzheimer’s Disease in a Patient Moreover, Alzheimer’s disease affects hypertrophies in the hippocampus as the initial part is involved in the brain’s memory areas and spatial orientation.
- Alzheimer’s Disease: Diagnostic and Treatment Alzheimer’s disease is a progressive degenerative disorder that causes a deterioration of mental and cognitive abilities.
- The Effect of Music on People With Alzheimer’s Disease The evidence suggests that one of the most prominent effects of music on patients with Alzheimer’s disease is autobiographical memory preservation alongside the stimulation of both sympathetic and parasympathetic nervous systems.
- Community Health: Alzheimer’s Disease The community nurse’s role is to develop and participate in primary, secondary, and tertiary preventive strategies and to provide a wide range of nursing care services while maintaining the health and wellbeing of individuals with […]
- Challenges of Living With Alzheimer Disease The medications make the condition of the patient better during the first stages of the disease. During the middle stage of the disease, the symptoms worsen.
- The Burden of Alzheimer’s Disease Assessing the appropriateness and effectiveness of reducing the cost of providing care for patients with Alzheimer remains a major issue that needs to be addressed.
- Chronic Care For Alzheimer’s Disease The application of the Chronic Care Model, in its turn, will serve as the foundation for building the patient’s awareness about their condition, thus, improving the patient’s quality of life and creating the environment, in […]
- Synopsis of Research Studies of Individuals Afflicted by Mild Alzheimer’s Disease The research questions in the articles were tailored along the various physical activities that can assist patients affected by Alzheimer Disease.
- Alzheimer’s Disease and Naturopathic Medicine The main feature of AD is the aggregation of -amyloid. However, application of natural therapies to prohibit the process of the pathways can slow the progress of AD.
- Brain Reduction and Presence of Alzheimer’s Disease The purpose of the study was to examine the correlation between brain reduction and the presence of Alzheimer’s disease. The researchers wanted to examine the nature of such changes in elderly individuals at low risk […]
- Alzheimer Related Morbidity and Death Among New Yorkers Generally, Alzheimer disease is a form of dementia, which inflicts a loss of memory, thinking and behavior. The proportion of ethnic and racial diversity in the US is increasing.
- Environmental Interview on a Patient With Alzheimer Disease In the 1980s, delusions and hallucinations were added as signs of the disease. Researches in the 1960’s show a link between cognitive reduction and the number of ailments in the brain.
- Alzheimer’s Disease Article and Clinical Trial This study shows that environmental hazards, in this case lead, increase the risk of developing Alzheimer’s disease and that the development period is crucial for determining future vulnerability to neurodegeneration and Alzheimer’s disease.
- Alzheimer’s Disease: Regarding Physiology However, one clear aspect of the development of this disease arises from a very complex chain of activities taking place in the brain over a long period of time.
- Mapping the Neurofibrillary Degeneration From Alzheimer’s Disease Patient This is an analytic review of the studies elaborating on the relationship of hyperphosphorylated tau proteins to the development of Alzheimer’s disease and focusing on the antigen capture ELISA specific for p-tau proteins.
- Role of Alzheimer’s Disease Advanced in Our Understanding of the Aging Process Aging on the hand can be defined as the accumulation of different harmful changes in the tissues and cells that raises the possibility of disease and death.
- Alzheimer’s Disease: Medical Analysis Such gene-associated markers have been characterized, in particular the apolipoprotein E gene, which was linked to chromosome# 19, and was responsible for accumulation of A by way of binding to this protein.
- Diabetic Teaching Plan for Alzheimer’s Patient He knows the purposes and some of the steps and needs to be taught again to regain his independence in monitoring his blood glucose level.
- Comparing Alzheimer’s Disease and Parkinson’s Disease There are many superficial similarities between Alzheimer’s disease and Parkinson’s disease primarily in some symptoms and age-group of persons afflicted by these two diseases.
- The Effects of Alzheimer’s Disease on Family Members The disease develops gradually and is said to be a disease of the old because it relates to the inability to remember.
- Alzheimer’s Disease in Science Daily News Article The news article accurately reports the focus of the study in the diagnosis of AD. Hence, the news article accurately presents that the diagnostic method is important in the diagnosis and prognosis of AD among […]
- Dancing and Risk of Alzheimer’s Disease Despite the fact that there is no effective treatment for Alzheimer’s disease, scientists discovered that dancing could help reduce the severity of the disorder as this activity involves simultaneous brain functioning, which helps to affect […]
- Alzheimer’s Disease Prevalence and Prevention The estimated global prevalence of Alzheimer’s disease is 50 million and is projected to triple by 2050 due to growth in the older generation. According to Alzheimer’s Association, AD is the fifth-ranking killer of persons […]
- Alzheimer’s and Cardiovascular Diseases Progress While the design of the study involves a review of the existing papers and a compilation of their key results, the information provided by the authors is nonetheless crucial to the understanding of the issue.
- The Alzheimer’s Disease Concept In simple words, it is the condition caused by the negative changes in the human brain that, as the end result, leads to memory loss and some behavioral issues that worsen the quality of patient’s […]
- Alzheimer’s Disease in Medical Research The existing data proposes that if the illness is distinguished before the commencement of evident warning signs, it is probable that the treatments founded on the facts of fundamental pathogenesis will be of assistance in […]
- Age Ailment: Dementia and Alzheimer’s Disease It is a time for one to clean the mind and take time to do what matters most in life. With an increased level of technological advancements, a digital sabbatical is mandatory to lower the […]
- Psychology Issues: Alzheimer’s Disease Alzheimer’s disease is a psychological disorder that involves the progressive destruction of brain cells and reduction in the proper functioning of the brain.
- Treatment of Alzheimer’s Disease According to documented research, Alzheimer’s disease is the primary cause of dementia affecting close to half a million people in the United Kingdom and five million in the United States.
- Health Care for Elderly People With Alzheimer’s Disease C’s condition is not likely to affect the relationship between her and her relatives if they are sensible toward her. C is to take her to a nursing home for the elderly.
- Diagnosis of Alzheimer’s Disease The most remarkable feature of the disease is the loss of ability to remember events in an individual’s life. According to the latter hypothetical medical study, it has been exemplified that the presence of deposits […]
- Concept and Treatment of the Alzheimer Disorder This implies that cognitive and natural therapies are highly perceived to be effective as opposed to pharmacological treatments. One cannot ignore the fact that both cognitive and natural therapies have become widely accepted in treating […]
- Understanding Alzheimer’s Disease Among Older Population After the 65 years, it has been found that the probability of developing Alzheimer’s disease doubles after every 5 years and as a result, by the age of 85 years, the risk of acquiring the […]
- Concepts of Alzheimer’s Disease The brain changes are the same in both men and women suffering from Alzheimer’s disease. There is also a significant increase in the death of the neurons leading to the shrinking of the affected regions.
- Alzheimer’s Association of Neurological Disorders and Stroke
- The Potential Treatment of Alzheimer’s Disease: Through CRISPR-Cas9 Genome Editing
- Alzheimer’s Condition as an Enemy of Mental Health
- Vitamin a as a Potential Therapy to Prevent Alzheimer’s Disease
- The Relationship Between Gender and Alzheimer’s Disease
- The Stages and Treatments of Alzheimer’s Disease
- The Clinical Description of the Causes, Symptoms and Treatment of Alzheimer’s Disease
- The Description of Alzheimer’s Disease and Its Statistics in America
- The Psychological Symptoms of Alzheimer’s the Cognitive Symptoms
- Varying Aspects of Alzheimer’s Disease and Implementations
- The Effects of Alzheimer’s and Dementia Among Elderly
- The Early Symptoms and Progression of Alzheimer’s Disease
- Watching a Loved One Slip Away From Alzheimer’s Disease
- The Differences Between Dementia and Alzheimer’s Dementia
- A History of Alzheimer’s Disease and Why It Is Still One of the Most Researched Diseases Today
- A Healthy Lifestyle Might Help Combat Parkinson’s Disease and Alzheimer’s Disease
- The Studies of Music and How It May Not Help the Alzheimer’s Disease
- The Trials of Caring for a Loved One With Alzheimer’s Disease
- Alzheimer’s Disease a Progressive and Fatal Disease of the Brain
- The Effects of Dementia and Alzheimer’s Disease on Caregivers and the Care Needed for Suffering Patients
- The Psychologist’s Role in Addressing Family and Community Problems for Families With Alzheimer’s Disease
- Alzheimer’s Disease and Its Effect on the Patient and Care Giver
- The Statistics of Prevalence of Alzheimer’s Disease in the 21st Century
- The Link Between Down Syndrome and Alzheimer’s Disease
- The Pathophysiology of Alzheimer’s Disease
- The Causes, Symptoms and Treatment of Alzheimer’s Disease
- The Focus on Alzheimer’s Disease in the Documentary “Black Daises for the Bride”
- The Physiology and Genetics Behind Alzheimer’s Disease
- The Early Manifestations of Alzheimer’s Disease
- The Role of Gamma Secretase in Alzheimer’s Disease
- The Lack of Early Detection of Alzheimer’s Disease
- The Representation of Alzheimer’s Disease and Its Impact in the Film “Still Alice”
- The Possible Link of the Human Immune System to Alzheimer’s Disease
- The Study of Alzheimer’s Disease and Its Affect on the Elderly
- The Characteristics, History, Symptoms, Statistics, and Treatment of Alzheimer’s Disease
- The Triggers, Progression, and Treatment of Alzheimer’s Disease
- Traumatic Brain Injury and Alzheimer’s Disease
- The Positive Impact of Exercise in Protecting the Brain From Alzheimer’s Disease
- Three Primary Types of Dementia: Alzheimer’s Disease, Vascular Dementia
- The Causes, Risks, Factors, and Stages of Alzheimer’s Disease
- The Contingent Valuation Method in Health Care: An Economic Evaluation of Alzheimer’s Disease
- What Is the Difference Between Dementia and Alzheimer’s Disease?
- What Is the Main Cause of Alzheimer’s Disease?
- How Do You Prevent Alzheimer’s Disease?
- Who Is at High Risk for Alzheimer’s Disease?
- What Foods Cause Alzheimer’s Disease?
- Do Alzheimer’s Disease Patients Sleep a Lot?
- Do Alzheimer’s Disease Patients Know They Have It?
- Do Alzheimer’s Disease Patients Feel Pain?
- What Is the Best Treatment for Alzheimer’s Disease?
- How Long Do Alzheimer’s Disease Patients Live?
- What Do Alzheimer’s Disease Patients Think?
- Do People with Alzheimer’s Disease Have Trouble Walking?
- Is End Stage Alzheimer’s Disease Painful?
- What Are the Final Stages of Alzheimer’s Disease Before Death?
- Does Alzheimer’s Disease Run in Families?
- Should You Tell Alzheimer’s Disease Patients the Truth?
- Why Do Alzheimer’s Disease Patients Stop Talking?
- How Do You Know When an Alzheimer’s Disease Patient Is Dying?
- Which Is Worse: Dementia or Alzheimer’s Disease?
- What to Say to Someone Who Has Alzheimer’s Disease?
- How Does Alzheimer’s Disease Affect Eyes?
- Are Alzheimer’s Disease Patients Happy?
- What Are the Warning Signs of Alzheimer’s Disease?
- What Is the Best Way to Help Someone with Alzheimer’s Disease?
- What Are Good Activities for Alzheimer’s Disease Patients?
- Disease Questions
- Disorders Ideas
- Nervous System Research Topics
- Pathogenesis Research Ideas
- Caregiver Topics
- Health Promotion Research Topics
- Neuropsychology Topics
- Therapeutics Research Ideas
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2024, November 16). 123 Alzheimer’s Disease Essay Topics & Examples. https://ivypanda.com/essays/topic/alzheimers-disease-essay-topics/
"123 Alzheimer’s Disease Essay Topics & Examples." IvyPanda , 16 Nov. 2024, ivypanda.com/essays/topic/alzheimers-disease-essay-topics/.
IvyPanda . (2024) '123 Alzheimer’s Disease Essay Topics & Examples'. 16 November.
IvyPanda . 2024. "123 Alzheimer’s Disease Essay Topics & Examples." November 16, 2024. https://ivypanda.com/essays/topic/alzheimers-disease-essay-topics/.
1. IvyPanda . "123 Alzheimer’s Disease Essay Topics & Examples." November 16, 2024. https://ivypanda.com/essays/topic/alzheimers-disease-essay-topics/.
Bibliography
IvyPanda . "123 Alzheimer’s Disease Essay Topics & Examples." November 16, 2024. https://ivypanda.com/essays/topic/alzheimers-disease-essay-topics/.
- Paper Writer Free >
- Essays Examples >
- Essay Topics >
Sample Research Paper On Alzheimer`s Disease
Type of paper: Research Paper
Topic: Medicine , Disease , Health , Alzheimer's Disease , Treatment , Aliens , Patient , Nursing
Published: 2021/02/12
Introduction
Alzheimer`s Disease is a chronic neurodegenerative disorder that is currently affecting an estimated 25 million people across the globe (Burock et al, 2014). The number of infection is expected to increase to approximately 75 million in the next 30 years. The disease is mostly experienced by elderly women in the age limit 65-85 (Solomon et al, 2014). However, in extreme ages such as 85+ years, the condition presents itself in pronounce levels. The prevalence of the disease is observed in both developed and underdeveloped nations. The disease is characterized by prominent psychiatric symptoms, memory impairment, and behavioral disturbances. These characteristics quite often limit an individual from carrying out the daily chores (Callaway, 2012).
Management of the Alzheimer’s Diseases
The initial step in managing AD is an accurate diagnosis of the disease, and then disclosing the issue in the most sensitive and timely manner. Research studies indicate that there is no known cause of the disease. However, the per-se conditions such as memory loss, behavioral disturbances and depression may act as vital elements to suspect the onset of the disease. A definitive psychological and laboratory tests is usually essential to confirm the existence of the condition (Solomon et al, 2014). The patient medical history family and co-morbidities will reveal the cause of the illness, affected cognitive domains, and the impact on the AD. Clinically, the general physical and neurological examination is particularly vital. According to the most recent expert opinions, they suggest the screening for the vitamin B12, Thyroid stimulating hormone, calcium, glucose and liver function abnormalities. It is also important to carry out serological tests for Borrelia, HIV and syphilis in circumstances where there are suggestive clinical features. These tests will help to distinguish the AD from other primary and secondary degenerative and dementia co-morbidities. Early diagnosis of the disease is important since it provides that patient and the family to get a better chance of treatment participate in advance research and plan for the future. Once the diagnosis is disclosed, Occupational therapy may be provided to the patient and the family (Callaway, 2012).
Treatment of the Alzheimer`s Disease
Alzheimer's Foundation of America in their research studies indicates that there is no approved cure for the AD. In most circumstances, clinicians have advised the use of Acetylcholinesterase inhibitors (donepezil, rivastigimine and galantamine). The Donepezil as an AChE inhibitor operates by accelerating the concentration of the acetylcholine at the sites of the body neurotransmitters. The drug should be administered at 5mg once per day at bedtime, and then increased to 10mg after one month if necessary. Galantamine also increases acetylcholine concentration at the neurotransmitter sites. It is often administered at 8mg per day. Rivestigimine also operates like the other two and administered at 1.5 mg twice per day. All these drugs operate by limiting the breakdown of acetylcholine, the essential messengers for memory and learning. By limiting the levels of acetylcholine, the drug support effective communication between the nerves (Solomon et al, 2014). However, it is important to note that these drugs possess varied side effects such as anxiety, constipation, headache, and dizziness. As a result of these adverse effects, there is insufficient evidence to support the application of any drug for treatment of the disease.
The Non-Pharmacologic Treatments
The Clinical meta-analysis indicates that, the non-pharmacological treatments are integral part of the overall treatment for the AD. These non-pharmacological treatments include; education, sensory stimulation, aromatherapy and personalized music. Patient caregivers should be equipped with relevant educational and support services necessary for the patient. Aromatherapy and sensory stimulation both alerts the nerves for maximum operation. Personalized music allows the patient to relax and achieve maximum nerve rest. The other most important option is to change the patient environment and eliminate the obstacles that are associated with the disease. Alzheimer's association report indicate that, identifying what triggers the disease is quite important while selecting the treatment approach (David et al, 2010).
Prevention of the Alzheimer`s Disease
Alzheimer's Foundation of America in their constant research has revealed that at current state, there is neither known specific cause nor treatment for the disease. Therefore, vigilant care is necessary to prevent the onset of the disease. In their quest, The Global Alzheimer's Association has identified some of the most common risk factors that are most likely to increase the development of the disease (Anderson et al, 2011). These include age, genetics and family history. Clinical intervention reveals that proper care should be taken to the memory especially at later ages. They further stipulate that people with lineage history of the disease should take much care by making regular checkups. The human head should be protected while operating any physical activity to prevent any form of injury (David et al, 2010).
Modern Research
Alzheimer's Foundation of America is on the constant move to advance the research on the AD. Although there are no known possible protective version of the gene to prevent AD, Clinical studies have discovered new test, drugs interventions that can detect, treat and prevent the disease in future. The studies are also searching for better methods of care and improved quality of life among the infected patients (Anderson et al, 2011).
As a result of the increased levels of the disease infection, it is important for everyone to provide a support in order to prevent further cases. The Global Alzheimer's Association should continue to provide their support and research on the best ways of handling emerging issues concerning the disease and its detrimental effects.
Anderson, L. N., McCaul, K. D., & Langley, L. K. (2011). Common-sense beliefs about the prevention of Alzheimer's disease. Aging & Mental Health, 15(7), 922-931. doi:10.1080/13607863.2011.569478 Alzheimer's association report finds 1 in 3 seniors dies with alzheimer's or another dementia. (2013). Professional Services Close – Up, 1-3 Burock, J., & Naquvi, L. (2014). Practical Management of Alzheimer's Dementia. Rhode Island Medical Journal, 97(6), 36-40. Callaway, E. (2012). Gene mutation defends against Alzheimer’s disease. Nature, 487, 153. Retrieved from http://www.nature.com/news/gene-mutation-defends-against-alzheimer-s-disease-1.10984 David, R., Zeitzer, J., Friedman, L., Noda, A., O'Hara, R., Robert, P., & Yesavage, J. (2010). Non-pharmacologic management of sleep disturbance in Alzheimer's disease. Journal Of Nutrition, Health & Aging, 14(3), 203-206. doi:10.1007/s12603-010-0050-9 Solomon, A., Mangialasche, F., Richard, E., Andrieu, S., Bennett, D. A., Breteler, M., & Kivipelto, M. (2014). Advances in the prevention of Alzheimer's disease and dementia. Journal Of Internal Medicine, 275(3), 229-250. doi:10.1111/joim.12178
Share with friends using:
- A Comparison Between Community Colleges And Universities Essays Example
- Doping Essays
- Gatherer Essays
- Neutrophil Essays
- Lymphocyte Essays
- Cranberry Essays
- Herbal Medicine Essays
- Borer Essays
- Atlantic Slave Trade Essays
- Hemp Essays
- Primates Essays
- Daimler Essays
- Araby Essays
- Bazaar Essays
- Polytheist Essays
- Quantification Essays
- Avalanche Essays
- Spur Essays
- El Nino Essays
- Cocktail Essays
- Bead Essays
- Brake Essays
- Harper Lee Essays
- Atticus Finch Essays
- Agatha Christie Essays
- Masterpiece Essays
- Complement Essays
- Sequel Essays
- Beard Essays
- Illuminati Essays
- Leprosy Essays
- Motorbike Essays
- Excursion Essays
- Medical Student Essays
- Book Of Job Essays
- Bookkeeping Essays
- Bernie Madoff Essays
- Economic Condition Essays
- Boston Massacre Essays
- Paul Revere Essays
- Crimea Crisis Essays
- Seljuk Essays
- Fortress Essays
- Viscose Essays
- Shadowing Essays
- Car Racing Essays
- Mongol Empire Essays
We use cookies to improve your experience with our site. Please accept before continuing or read our cookie policy here .

Wait, have you seen our prices?
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Alzheimer’s Disease: Past, Present, and Future
Mark w bondi, emily c edmonds, david p salmon.
- Author information
- Copyright and License information
Correspondence and reprint requests to: Mark W. Bondi, University of California San Diego, 9500 Gilman Drive, La Jolla, CA 92093-0603V. [email protected]
Although dementia has been described in ancient texts over many centuries (e.g., “Be kind to your father, even if his mind fail him.” – Old Testament: Sirach 3:12), our knowledge of its underlying causes is little more than a century old. Alzheimer published his now famous case study only 110 years ago, and our modern understanding of the disease that bears his name, and its neuropsychological consequences, really only began to accelerate in the 1980s. Since then we have witnessed an explosion of basic and translational research into the causes, characterizations, and possible treatments for Alzheimer’s disease (AD) and other dementias. We review this lineage of work beginning with Alzheimer’s own writings and drawings, then jump to the modern era beginning in the 1970s and early 1980s and provide a sampling of neuropsychological and other contextual work from each ensuing decade. During the 1980s our field began its foundational studies of profiling the neuropsychological deficits associated with AD and its differentiation from other dementias (e.g., cortical vs . subcortical dementias). The 1990s continued these efforts and began to identify the specific cognitive mechanisms affected by various neuropathologic substrates. The 2000s ushered in a focus on the study of prodromal stages of neurodegenerative disease before the full-blown dementia syndrome (i.e., mild cognitive impairment). The current decade has seen the rise of imaging and other biomarkers to characterize preclinical disease before the development of significant cognitive decline. Finally, we suggest future directions and predictions for dementia-related research and potential therapeutic interventions.
Keywords: Neuropsychology, Cognition, Neuroscience, Alzheimer’s disease, Mild cognitive impairment, Neuroimaging, Biomarkers, Clinical trials
INTRODUCTION
One of the great challenges faced by neuropsychologists over the past 50 years is to understand the cognitive and behavioral manifestations of dementia and their relationship to underlying brain pathology. This challenge has grown substantially over the years with the aging of the population and the age-related nature of many dementia-producing neurodegenerative diseases. Although the concept of dementia has existed for thousands of years ( Mahandra, 1984 ), it is only early in the past century that the essential clinical syndrome and associated neurodegenerative changes were first discovered. In 1907, Aloysius “Alöis” Alzheimer carefully described the symptoms of a 51-year-old woman, Auguste Deter, who was under his care at the state asylum in Frankfurt Germany ( Alzheimer, 1907 ; for an English translation, see Stelzmann et al., 1995 ) ( Figure 1 ). Alzheimer’s description of her symptoms is almost certainly the first neuropsychological characterization of the disease:
“ Her memory is seriously impaired. If objects are shown to her, she names them correctly, but almost immediately afterwards she has forgotten everything. When reading a test, she skips from line to line or reads by spelling the words individually, or by making them meaningless through her pronunciation. In writing she repeats separate syllables many times, omits others and quickly breaks down completely. In speaking, she uses gap-fills and a few paraphrased expressions (“milk-pourer” instead of cup); sometimes it is obvious she cannot go on. Plainly, she does not understand certain questions. She does not remember the use of some objects .”
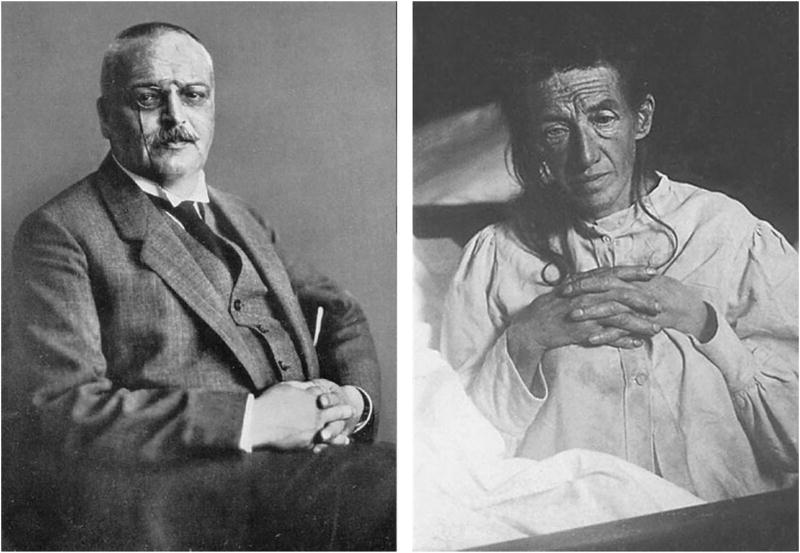
Photographs of Alois Alzheimer (left) and his patient Auguste Deter (right).
When Auguste Deter died, Alzheimer used the then-new silver staining histological technique to examine her brain microscopically. When he did so, he observed the neuritic plaques, neurofibrillary tangles, and amyloid angiopathy that were to become the hallmarks of the disease that now bears his name (as shown in Figure 2 from sketches of the histologic preparations in his 1911 paper). Alzheimer himself did not claim to have discovered “Alzheimer’s disease,” although his mentor Emil Kraepelin at the Munich Medical School rightly credited him with doing so by coining the term in his own Handbook of Psychiatry ( Kraepelin, 1910 ). By 1911, the medical community was using Alzheimer’s depictions of the disease to diagnose patients both in Europe and the United States (Mauer & Mauer, 2003).
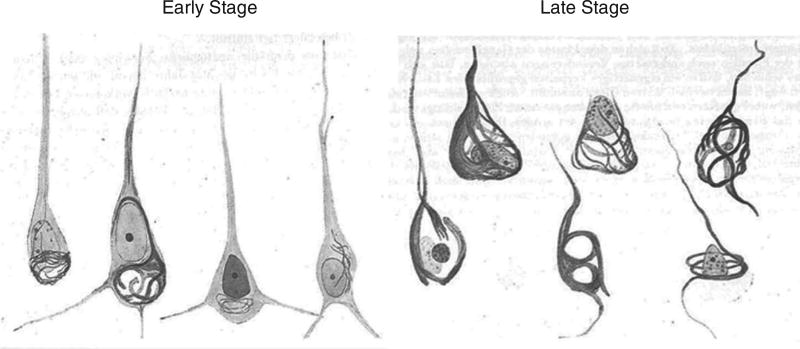
Sketches of Auguste Deter’s histopathologic preparations of early and late stage neurofibrillary tangle pathology as drawn by Alzheimer from his 1911 paper entitled “Über eigenartige Krankheitsfälle des späteren Alters.”
It was also during this time that Eugen Blueler in his study of schizophrenia coined the term “organic psychosyndrome” to refer to decrements in memory, judgment, perceptual discrimination and attention, emotional lability, and defective impulse control associated with chronic diffuse cortical damage. This classification was essentially adopted by the American Psychiatric Association (APA) to define dementia in the first two editions of their Diagnostic and Statistical Manual of Mental Disorders (DSM). Specifically, DSM-II defined “organic brain syndrome” as a “basic mental condition characteristically resulting from diffuse impairment of brain tissue function from whatever cause,” and which is manifested behaviorally as impairment in orientation, memory, intellectual functions, judgment, and affect ( APA, 1968 ).
Armed with these uniform criteria and newly developed standardized bedside cognitive screening tests ( Blessed, Tomlinson, & Roth, 1968 ; Folstein, Folstein, & McHugh, 1975 ), a handful of investigators began scientific studies of dementia, particularly focusing on dementia associated with Alzheimer’s disease (AD). Although dementia is associated with more than 70 different causes of brain dysfunction, AD is the most common cause accounting for roughly half of all cases (for review, see Cummings & Benson, 1992 ). One of the most important studies during this period showed that the degree of AD pathology in the brain was significantly correlated with performance on standardized cognitive tests shortly before death ( Blessed, Tomlinson, & Roth, 1968 ). This was the first study to strongly link the clinical features of AD with the pathologic brain changes that Alzheimer had described ( Figure 3 ).
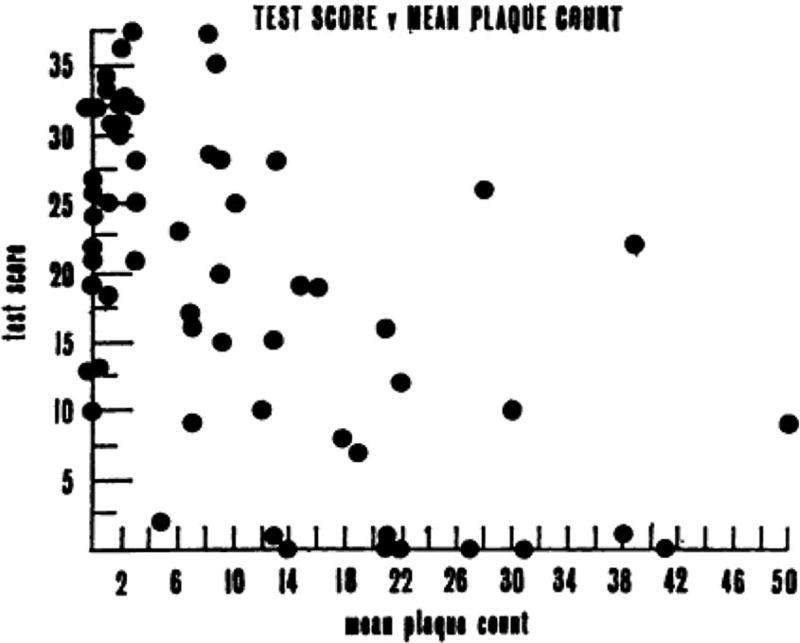
Mean plaque count plotted against the summary cognitive test score constructed by Blessed, Tomlinson, & Roth (1968) . The “Blessed” test score was computed from “a number of simple psychological tests of orientation, remote memory, recent memory, and concentration,” resulting in a total score ranging from 0 (complete failure) to 37 (perfect score). The scatterplot resulted in a highly significant correlation coefficient of −0.59 ( p <.001) (from Blessed, Tomlinson, & Roth, 1968 ).
Neuropsychological studies of dementia and AD during this period were rare and largely limited to presenile dementia with onset before the age of 65. A notable exception was a series of studies by Edgar Miller who showed that the main behavioral feature of presenile AD is a memory disorder in which recently acquired information fails to reach long-term memory storage due to both an abnormally rapid loss of material from short-term storage (perhaps due to encoding inefficiency) and difficulty in transferring information between short-term and long-term storage systems ( Miller, 1971 , 1973 ). He also suggested that inefficient retrieval of information from long-term storage may contribute to the memory deficit in presenile AD ( Miller, 1975 , 1978 ). These early studies set the stage for countless subsequent studies that examined the nature of memory dysfunction in AD in the decades to follow (for reviews, see Salmon & Bondi, 2009 ; Smith & Bondi, 2013 ).
A major sea-change in the study of dementia occurred in 1976 when Robert Katzman summarized data showing that senile and presenile AD were histopathologically identical and suggested that, based on epidemiological data, AD was the fourth leading cause of death in the elderly ( Katzman, 1976 ). Suddenly, AD dementia went from a relatively rare condition to a major public health issue. This led to greater attention to the disease by the public and at the National Institutes of Health, which established the National Alzheimer’s Disease Research Center program to study the cause, neuropathology, and clinical characteristics of AD. At this time, the diagnostic criteria for dementia were refined in the DSM-III ( American Psychiatric Association, 1980 ) and International Statistical Classification of Diseases and Related Health Problems, 10th Revision ( World Health Organization, 1992 ), and specific research diagnostic criteria for AD were established ( McKhann et al., 1984 ).
Also notable at this time was a growing realization that various dementing disorders are associated with patterns of relatively preserved and impaired cognitive abilities that vary depending upon the etiology and neuropathology of the underlying disease. Martin Albert and his colleagues ( Albert, Feldman, & Willis, 1974 ) referred to the pattern of cognitive dysfunction observed in patients with progressive supranuclear palsy as a “subcortical dementia” characterized by forgetfulness, slowness of thought processes, altered personality with apathy or depression, and impaired ability to manipulate acquired knowledge. Similar cognitive changes were noted in patients with Huntington’s disease ( McHugh & Folstein, 1975 ). This pattern of impairment was contrasted with the cortical dementia (e.g., frank amnesia, aphasia, and agnosia) observed in AD. Subsequent studies further delineated qualitative differences in the cognitive deficits associated with so-called “cortical” and “subcortical” dementing disorders ( Huber, Shuttleworth, Paulson, Bellchambers, & Clapp, 1986 ; Salmon, Kwo-on-Yuen, Heindel, Butters, & Thal, 1989 ), and several investigators suggested that these two forms of dementia should be recognized as distinct clinical syndromes (for reviews, see Cummings & Benson, 1992 ; Cummings, 1990 ).
THE 1990s: NEUROPSYCHOLOGICAL CHARACTERIZATION OF ALZHEIMER’S DISEASE AND RELATED DISORDERS
The new criteria for dementia and AD adopted in the 1980s improved the reliability of the clinical diagnosis and allowed group studies of mildly demented patients to be carried out with a reasonable degree of accuracy. Many of these studies applied the theories and methods of cognitive psychology to study the cognitive consequences of AD. By using this approach, these studies characterized the component cognitive processes underlying the neuropsychological deficits observed in AD, and showed that cognitive changes attributable to AD and other dementing disorders could have important implications for existing theories of brain–behavior relationships underlying normal cognition.
Several studies at this time showed that episodic memory impairment (i.e., amnesia) is usually the earliest and most salient aspect of the AD dementia syndrome. These findings were consistent with neuropathologic studies that showed extensive AD pathology occurs earliest in medial temporal lobe (MTL) structures (e.g., hippocampus, entorhinal cortex) important for episodic memory ( Hyman et al., 1984 ). The memory deficit was shown to reflect an inability to effectively encode and store new information since patients with very early AD were particularly impaired on measures of delayed recall (i.e., have abnormally rapid forgetting), exhibited an abnormal serial position effect with attenuation of the primacy effect (i.e., recall of words from the beginning of a list), and remained impaired even if retrieval demands were reduced by the use of recognition testing (e.g., Delis et al., 1991 ).
Semantic encoding was found to be less effective in improving the episodic memory performance of patients with AD than normal elderly individuals ( Buschke, Sliwinski, Kuslansky, & Lipton, 1997 ). In addition, patients with AD more often produced intrusion errors (i.e., previously learned information is produced during the attempt to recall new material) on both verbal and non-verbal memory tests, presumably due to increased sensitivity to interference and/or decreased inhibitory processes ( Butters, Granholm, Salmon, Grant, & Wolfe, 1987 ; Jacobs, Salmon, Tröster, & Butters, 1990 ). This pattern of memory deficits was shown to differ from the pattern exhibited by patients with subcortical dementia who had difficulty learning new information, but retained what was learned well and showed improved performance with retrieval aids (e.g., cueing or recognition formats) ( Cummings, 1990 ). These findings provided evidence of differential roles of MTL and fronto-striatal brain structures in memory performance.
Studies also showed that, as the neuropathology of AD spreads beyond MTL structures to adjacent temporal, parietal, and frontal association cortices, several higher order cognitive abilities became affected. A deficit in language abilities (i.e., aphasia) was observed relatively early in the course of AD, with deficits in confrontation naming, verbal fluency (particularly from semantic categories), semantic categorization, and a reduced ability to recall over-learned facts (e.g., the number of days in a year) ( Hodges & Patterson, 1995 ; Nebes, 1989 ). Patients were highly consistent in the individual items they missed across different semantic memory tests that used unique modes of access and output (e.g., fluency versus confrontation naming; Chertkow & Bub, 1990 ; Hodges, Salmon, & Butters, 1992 ), or within the same test across serial evaluations ( Norton, Bondi, Salmon, & Goodglass, 1997 ).
These findings demonstrated that AD results in a true loss of semantic knowledge (i.e., general knowledge and the meanings of words) rather than only an impaired ability to retrieve information from intact semantic memory stores (also see Salmon, Heindel, & Lange, 1999 ). A similar loss of knowledge was thought to contribute to the severe deficit patients with AD exhibited in the ability to remember past events that were successfully remembered before the onset of the disease (i.e., retrograde amnesia) ( Squire, 1987 ). Patients with subcortical dementia or fronto-temporal dementia, in contrast, retained semantic knowledge well, but had difficulty in systematic retrieval from semantic memory stores ( Rosser & Hodges, 1994 ; Rascovsky, Salmon, Hansen, Thal, & Galasko, 2007 ).
Deficits in “executive” functions responsible for concurrent mental manipulation of information, concept formation, problem solving, and cue-directed behavior were found to develop in the course of AD ( Bondi, Monsch, Butters, Salmon, & Paulsen, 1993 ; Lefleche & Albert, 1995 ; Perry & Hodges, 1999 ). Attention deficits were also found to occur and were usually evident on dual-processing tasks, tasks that require the disengagement and shifting of attention, and working memory tasks that depend upon the control of attentional resources (for reviews, see Parasuraman & Haxby, 1993 ; Perry & Hodges, 1999 ). Deficits in working memory were relatively mild and primarily characterized by disruption of the “central executive” with relative sparing of immediate memory ( Baddeley, Bressi, Della Sala, Logie, Spinnler, 1991 ; Collette, Van der Linden, Bechet, Salmon, 1999 ). Executive dysfunction and deficits in attention played a less prominent role in the AD dementia syndrome than in the subcortical dementia syndrome associated with fronto-striatal dysfunction.
Several studies showed that visuospatial deficits occurred in patients with AD (for review, see Cronin-Golomb & Amick, 2001 ), but these deficits were usually less salient than other cognitive deficits in the early stages of the disease ( Storandt Botwinick, Danziger, Berg, Hughes, 1984 ). Visuospatial tasks that were sensitive to early AD often involved not only visuoperceptual and constructional aspects of performance, but also required conceptual knowledge (e.g., Clock Drawing; Rouleau, Salmon,, Butters, Kennedy, & McGuire, 1992 ) or planning ability (e.g., Block Design).
The advances made in characterizing the neuropsychological deficits associated with AD had a major impact on the ability to accurately diagnose the disease in its early stages. This clinical utility was demonstrated in a study that compared the ability of several sensitive measures of learning and memory, executive abilities, language, and visuospatial abilities to differentiate between mild AD and matched normal control subjects ( Salmon et al., 2002 ). Results showed excellent sensitivity and specificity for learning and delayed recall measures from the California Verbal Learning Test (CVLT) (sensitivity: 95–98%, specificity: 88–89%), category fluency (sensitivity: 96%, specificity: 88%), and Trail-Making Part B (sensitivity: 85%, specificity: 83%). The best-fitting combination of category fluency and delayed recall accurately classified 96% of the patients with AD and 93% of the control subjects (see Figure 4 ). This study also illustrated that the pattern of cognitive deficits typically associated with AD is characterized by prominent deficits in episodic and semantic memory, with additional, although somewhat less prominent, deficits in executive functions, visuospatial abilities, and attention.
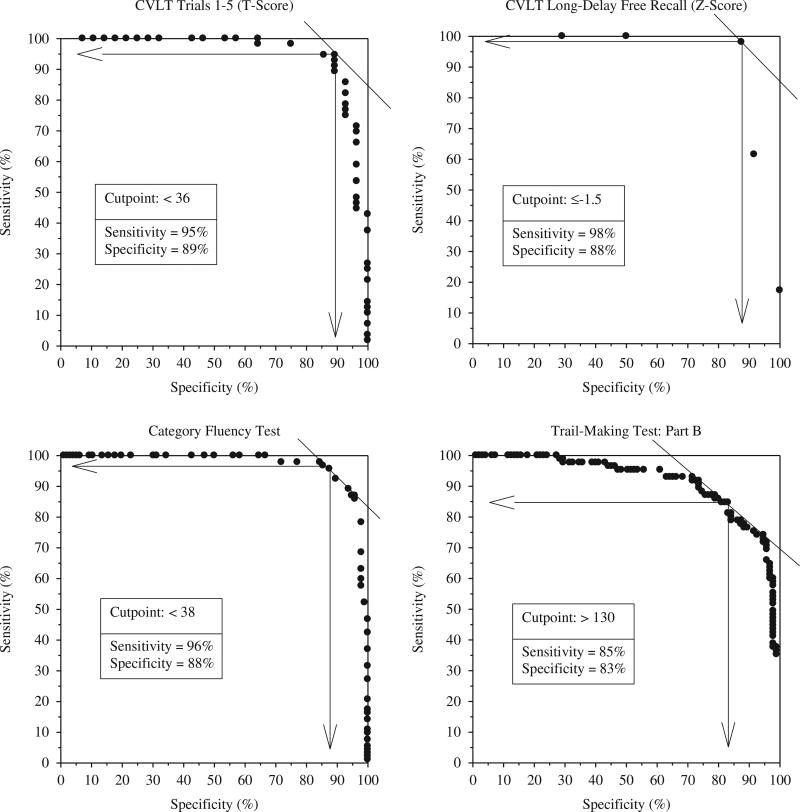
Receiver operating characteristic curves demonstrating excellent sensitivity and specificity for the accurate diagnosis of early AD achieved with neuropsychological tests of memory (California Verbal Learning Test), language (category fluency: animals, fruits, and vegetables) and executive functions (Trail-Making Test: Part B) (adapted from Salmon et al., 2002 ).
There are, however, somewhat rare instances, particularly in younger patients (e.g., less than 65 years old), where AD initially presents with dementia dominated by higher-order visual dysfunction, executive dysfunction or deficits in language. Posterior cortical atrophy (PCA) occurs when there is disproportionate atrophy and deposition of neurofibrillary tangles and neuritic plaques in the occipital cortex and posterior parietal cortex relative to other cortical association areas ( Hof, Vogt, Bouras, & Morrison, 1997 ; Renner et al., 2004 ). Patients with PCA usually have prominent visual agnosia (sometimes including prosopagnosia) and constructional apraxia, and exhibit many or all of the features of Balint’s syndrome, including optic ataxia, gaze apraxia, and simultanagnosia (i.e., can detect visual details of an object but cannot organize them into a meaningful whole) ( Caine, 2004 ; Mendez et al., 2002 ; Renner et al., 2004 ). PCA is associated with posterior cortical hypometabolism with particular involvement of the dorsal visual stream ( Nestor, Caine, Fryer, Clarke, & Hodges, 2003 ), and with a posterior distribution of amyloid deposition revealed by positron emission tomography (PET) imaging using Pittsburgh compound-B ([ 11 C]-PIB) ( Tenovuo, Kemppainen, Aalto, Nagren, & Rinne, 2008 ).
A frontal variant of AD was identified in a subgroup of patients with autopsy-confirmed AD who initially presented with disproportionately severe deficits on neuropsychological tests of frontal lobe functioning ( Johnson, Head, Kim, Starr, & Cotman, 1999 ). These patients had a significantly higher burden of neurofibrillary tangles, but not neuritic plaques, in the frontal cortex than a matched group of patients with a typical clinical presentation of AD. A subset of patients with primary progressive aphasia (PPA) was found to have AD pathology. These patients usually presented with logopenic PPA (PPA-L), which is characterized by hesitant, grammatically correct speech and spared language comprehension ( Gorno-Tempini et al., 2004 ). PPA-L is most often associated with AD pathology disproportionately distributed in language-related cortical areas ( Mesulam et al., 2008 ).
The existence of these AD “variants” has complicated the clinical and neuropsychological differentiation of AD from other neurodegenerative diseases that may have a different underlying focal pathology such as frontotemporal lobe dysfunction (FTLD), dementia with Lewy bodies (DLB), or PPA. However, considerable work has been done to identify how the neuropsychological presentations of these disorders differs from that of typical AD, and this information has been incorporated into the most recent clinical diagnostic criteria for behavioral variant FTLD ( Rascovsky et al., 2011 ), DLB ( McKeith et al., 2017 ), and PPA ( Gorno-Tempini et al., 2011 ).
During the 1990s and early 2000s, important advances were also made in identifying genetic risks for AD. Mutations on three separate genes were identified in large families that displayed an autosomal dominant inheritance pattern of an early-onset form of AD (i.e., onset generally before the age of 60): the amyloid precursor protein gene on chromosome 21, the presenilin 1 gene on chromosome 14, and the presenilin 2 gene on chromosome 1 (for review, see Bird, 1999 ). These forms of familial AD are rare and account for only approximately 1 to 2% of all cases of the disease. A far more common genetic risk factor for sporadic, late-onset AD was identified as the type ε4 allele of the gene for apolipoprotein E (APOE), a low density lipoprotein cholesterol carrier ( Strittmatter et al., 1993 ). Located on chromosome 19, the APOE ε4 allele was found to be present in 50 to 60% of patients with AD (compared to 20 to 25% of healthy older adults), regardless of whether or not they have a family history of dementia ( Strittmatter, et al., 1993 ). Unlike the genes associated with early-onset familial AD, the APOE ε4 allele is not deterministic, but confers an approximately three-fold risk of developing AD if one copy of the ε4 allele is present, and an eight-fold risk if two copies are present ( Katzman & Kawas, 1994 ).
The identification of the APOE ε4 risk led to a new approach to examining potential decrements in learning and memory during a “preclinical” phase of AD. The performance of non-demented older adults who have an increased risk for developing the disease due to an APOE ε4 genotype could be compared to that of individuals who do not have this risk factor with the presumption that more individuals with the ε4 genotype are in a preclinical stage of the disease. In one such study, Bondi, Salmon, Galasko, Thomas, and Thal (1999) compared the neuropsychological test performances of non-demented elderly individuals with or without at least one APOE ε4 allele. Although the groups did not differ significantly in age, education, or global cognitive status, the ε4+ subjects performed significantly worse than the ε4− subjects on measures of delayed recall, but not on tests of other cognitive abilities.
Cox proportional hazards analysis showed that APOE ε4 status and measures of delayed recall were significant independent predictors of subsequent progression to AD, suggesting that poor recall is an early sensitive neuropsychological marker of AD and not a cognitive phenotype of the ε4 genotype (also see Bondi et al., 1995 ; Petersen et al., 1995 ; Reed et al., 1994 ). Although ApoE remains the most potent susceptibility gene, the advent of genome wide association studies have identified 25 loci known to associate with late-onset sporadic AD, and the advent of polygenic risk scores are now available and will further refine our understanding of genetic contributions to AD progression (for review, see Sims & Williams, 2016 ).
THE 2000s: “MILD COGNITIVE IMPAIRMENT”
Although in the 1990s a few investigators had begun to systematically study individuals at risk for dementia to determine whether cognitive declines could be detected before diagnosis ( Bondi et al., 1994 , 1999 ; La Rue, Matsuyama, McPherson, Sherman, & Jarvik, 1992 ; Small, Fratiglioni, Viitanen, Winblad, & Bäckman, 2000 ; Snowdon et al., 1996 ), following the turn of this century, the focus of the field heavily shifted to the study of prodromal stages of AD that precede the full-blown dementia syndrome. Characterization of such early phases was largely crystallized by Ron Petersen, Glenn Smith, and colleagues from the Mayo Clinic who introduced of the concept of “mild cognitive impairment” (MCI) ( Petersen et al., 1999 ).
MCI was defined as a condition in which individuals experience memory loss to a greater extent than one would expect for age, yet do not meet criteria for dementia. The specific clinical criteria for MCI they originally put forth were: (1) subjective memory complaint, (2) objective memory impairment for age, (3) relatively preserved general cognition, (4) essentially intact activities of daily living, and (5) not demented ( Petersen et al., 1999 ). This classification scheme was subsequently broadened to include “amnestic MCI” or “non-amnestic MCI” subtypes, and “single domain” or “multiple domain” conditions to indicate the number of cognitive domains affected ( Petersen, 2004 ; Winblad et al., 2004 ). It was proposed that these MCI subtypes correspond to various etiologies, with “amnestic MCI” being most indicative of AD and “non-amnestic MCI” suggesting other neurodegenerative conditions such as FTLD or DLB ( Petersen & Morris, 2005 ; see also Smith & Bondi, 2013 , for review).
With the advent of these new criteria, the study of MCI became widespread during the 2000s. To illustrate this increasing attention and productivity, Petersen and colleagues (2009) noted that in 1999 fewer than 50 papers were published in the medical literature on the topic of MCI, whereas by 2007, this number approached 900 peer-reviewed studies in that year alone (see Figure 5 ). He rightly concluded that the increased awareness and study of MCI had been extremely valuable for the field by enhancing our understanding of the early neuropsychological manifestations of AD and improving the ability to identify those at risk for progression to dementia.
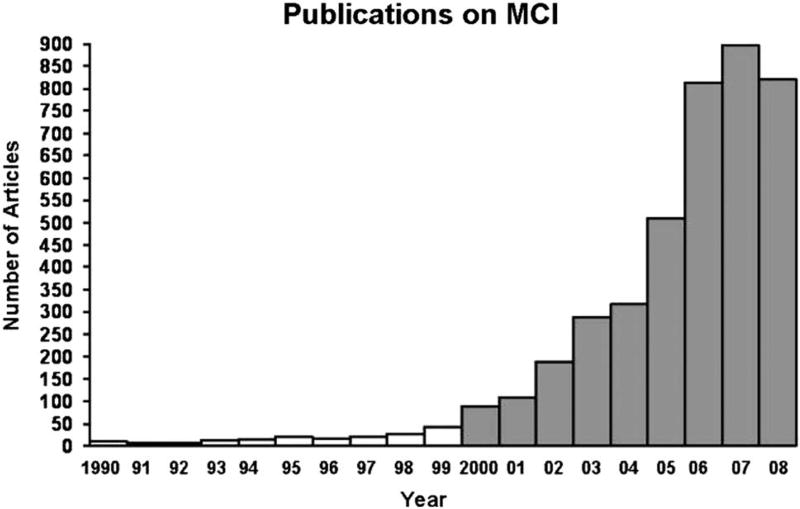
A representative number of publications with the search term “mild cognitive impairment” in the title or abstract from 1990 through part of 2008. Note the exponential rate of increase in the numbers of publications during the 2000s (from Petersen et al., 2009 ).
Detection and characterization of prodromal AD continued to be a vibrant area of research moving into the 2010s. In 2011, the National Institute on Aging and Alzheimer’s Association (NIA-AA) published updated diagnostic guidelines for MCI ( Albert et al., 2011 ) and introduced research criteria for “preclinical” AD ( Sperling et al., 2011 ). The new guidelines for MCI largely retained the criteria developed by Petersen and colleagues, but expanded the subjective cognitive complaint criterion to allow the complaint to come from either the patient, an informant or a skilled clinician, and incorporated the use of biomarkers into the diagnosis (discussed below). Research began on the potential of subjective cognitive complaints alone to accurately signal the development of underlying AD pathology (for review, see Jessen et al., 2014 ). Criteria for “preclinical” AD were developed to identify at-risk individuals at a stage of disease when they were still considered “asymptomatic” (i.e., had no significant cognitive impairment in the presence of one or more positive biomarkers for AD).
Although the criteria for MCI have been widely adopted, recent research has demonstrated limitations in the way the criteria were operationalized for clinical trials (e.g., Petersen et al., 2005 ) and large-scale natural history studies (e.g., the Alzheimer’s Disease Neuroimaging Initiative or ADNI; Weiner et al., 2013 ). These studies operationalized MCI as subjective complaints about memory, normal performance on simple cognitive screens, marginal memory ratings on scales based on clinical judgment, and impaired performance on a single memory test. Unfortunately, this method appears to be highly susceptible to false positive diagnostic errors ( Bondi et al., 2014 ; Clark et al., 2013 ; Edmonds, Delano-Wood, Clark, et al., 2015 ).
This susceptibility was demonstrated by Edmonds, Delano-Wood, Clark, et al. (2015) who applied cluster-analytic statistical techniques to the neuropsychological test scores of participants in the ADNI cohort who had been classified as MCI using the conventional criteria. Despite their MCI diagnosis , approximately one-third of these participants performed within normal limits on this more extensive cognitive testing and showed a low rate of progression to dementia. Given these limitations in the conventional diagnostic criteria, Jak, Bondi, and colleagues ( Jak et al., 2009 , Bondi et al., 2014 ) developed an actuarial neuropsychological diagnostic method to identify individuals with MCI. Rather than using a single memory test, a diagnosis of MCI is established on the basis of scores achieved on multiple objective neuropsychological tests that assess a range of cognitive domains without reference to subjective complaints or clinical judgment. This actuarial method was shown to produce greater diagnostic stability than the conventional method (i.e., individuals classified as MCI did not revert to “normal” cognition after 1 year; Jak et al., 2009 ), and revealed stronger relationships between cognition, biomarkers, and rates of progression to dementia in patients classified as MCI in this way ( Bondi et al., 2014 ).
THE 2010s: THE ERA OF BIOMARKERS
Over the past 20 years great progress was made in identifying in vivo biological markers of AD. Several investigators refined the ability to detect and measure cerebrospinal fluid levels of Aβ (the main constituent of the plaque) and tau protein (a constituent of the neurofibrillary tangle) that were indicative of AD pathology in the brain. Klunk and colleagues (see Mathis et al., 2003 ) developed Pittsburgh compound-B ([ 11 C]-PIB), an agent that binds to Aβ, for use with PET imaging to reveal deposition of amyloid in the brain. Tau-binding agents that can be used with PET imaging have also been recently developed (for review, see Brosch, Farlow, Risacher, & Apostolova, 2017 ).
Neuroimaging measures of hippocampal, cortical, and general brain atrophy were developed and applied to detect early neurodegenerative changes associated with AD (for review, see Frisoni, Fox, Jack, Scheltens, & Thompson, 2010 ). Other advanced structural and functional neuroimaging methodologies, including resting-state functional MRI and diffusion tensor imaging, have been used to detect pathological changes associated with AD and to create algorithms for classifying AD and MCI (for review, see Rathore, Habes, Iftikhar, Shacklett, & Davatzikos, 2017 ). All of these biomarkers have greatly increased the accuracy with which AD pathology in the brain can be detected before the onset of cognitive symptoms, and improved the ability to differentiate AD from other pathologies that lead to dementia.
In the current decade, several large-scale longitudinal studies have examined the relationship between various AD biomarkers and the development of cognitive decline and dementia (e.g., ADNI, Australian Imaging, Biomarkers, and Lifestyle study). Based on results from these studies, Jack and colleagues (2010) proposed a hypothetical model of dynamic biomarker changes in the development of AD. Their model, consistent with the amyloid cascade hypothesis , proposed that amyloid deposition related to abnormal processing of the amyloid precursor protein (i.e., amyloidosis) drives the formation of abnormal tau aggregates. This in turn leads to tangle-mediated neuronal injury and neurodegeneration, which then produces cognitive and functional impairment (see Jack et al., 2010 , 2013 , for discussion).
Many biomarker studies align with this temporal sequence of pathophysiologic changes, particularly in early-onset autosomal dominant mutation carriers (e.g., Bateman et al., 2012 ). The model has been very influential in the development of treatment strategies for AD because it posits that, if the preclinical build-up of amyloid can be blocked or built-up, amyloid can be cleared and the cascade of events that leads to cognitive decline and dementia can be prevented (for review, see Musiek & Holtzman, 2015 ). The hypothesis also provided the framework for revised diagnostic criteria for AD ( McKhann et al., 2011 ), MCI ( Albert et al., 2011 ), and preclinical AD ( Sperling et al., 2011 ).
Despite its wide influence, there is increasing evidence that calls the amyloid cascade hypothesis into question, especially with regard to its invariant temporal sequence of pathological events ( Drachman, 2014 ). Several studies, for example, have shown that neurodegeneration (measured by tau biomarkers or neuroimaging measures of atrophy) can occur before amyloidosis in individuals with prodromal AD ( Braak, Zetterberg, Del Tredici, & Blennow, 2013 ; Knopman et al., 2013 ; Ryan et al., 2013 ; Sheline et al., 2010 ; Wirth et al., 2013 ). Neurodegeneration in the face of normal amyloid levels was evident in 23% of the original sample of Jack et al. (2010) (and in an even higher percentage in Edmonds, Delano-Wood, Galasko, et al., 2015 ). Axonal injury ( Ryan et al., 2013 ) and tau lesions in late-myelinating regions ( Braak et al., 2011 ) have been shown to predate amyloid deposition in prodromal AD.
In addition, a growing number of studies have shown that cognitive measures can be as sensitive as physical biomarkers in predicting progression to dementia ( Gomar et al., 2014 ; Heister et al., 2011 ; Jedynak et al., 2012 ; Landau et al., 2010 ; Richard, Schmand, Eikelenboom, Van Gool; Alzheimer’s Disease Neuroimaging Initiative, 2013 ). Taken together, these findings strongly suggest that the neurodegeneration of AD may not depend upon prior amyloidosis ( Knopman et al., 2013 , but cf. Jack, Knopman, et al., 2016 ).
Our prior work ( Edmonds, Delano-Wood, Galasko, et al., 2015 ) in this area confirms that biomarker development in most individuals with preclinical/prodromal AD does not follow the temporal order proposed by the amyloid cascade hypothesis. We have shown that cognitively normal individuals who later progressed to MCI or AD, and had only one abnormal biomarker at baseline, were most likely to have neurodegeneration (i.e., P-tau positivity) as that abnormal biomarker rather than either amyloidosis alone or subtle cognitive deficit alone. In fact, neurodegeneration in isolation was 2.5 times more common than amyloidosis alone.
Jack, Bennett, and colleagues (2016) have recently acknowledged these and similar findings and proposed a more descriptive classification scheme for AD biomarkers that is agnostic to the temporal ordering of mechanisms underlying AD pathogenesis. This new model, known as the A/T/N system (“A” refers to Aβ, “T” to tau, and “N” to neurodegeneration), makes no assumptions about temporal ordering of biomarkers or their putative causal relationships. This “agnosticism” concurs with the notion of a simple tallying of biomarker risks as previously suggested by Edmonds, Delano-Wood, Galasko, et al. (2015) .
Such a dramatic shift away from the strictures of the amyloid cascade model toward a more equipotential conceptualization of AD biomarker risks espoused by our tally system and by the A/T/N classification system fits well with a continuum hypothesis proposed by Braak and colleagues. An original Braak staging theory proposed that progression of neurofibrillary tangle pathology proceeds along well-defined predilection sites beginning in the MTL and then expands to adjacent association cortices and beyond ( Braak & Braak, 1991 ). Amyloid plaque pathology, in contrast, accumulates more diffusely across neocortex.
This theory was recently updated to suggest that the pathogenic process actually starts with the formation of pretangle material in the lower brainstem with the first visible pathologic changes occurring in the locus coeruleus ( Braak et al., 2011 ). Tangle pathology then spreads (possibly through cell-to-cell propagation; Iba et al., 2015 ) to MTL through specific projections from the locus coeruleus. It is postulated that this begins well before amyloidosis. Braak and Del Tredici (2015) proposed that the initial tau pathology in locus coeruleus and its axonal projections may not result in outright neuronal death, but may restrict neuronal function. Thus, a central role of neuropsychology in the coming decades may be to provide sophisticated measurement of functionality of affected neural systems in preclinical/prodromal AD.
Critics of the continuum theory argue that tau aggregation confined to brainstem structures and MTL, in the context of little to no amyloid deposition, should be considered an independent pathological process that is not integral to the developmental continuum of sporadic AD. They have termed this condition primary age-related tauopathy (PART; Crary et al., 2014 ). In this view, the pathological diagnosis of AD requires the presence of amyloid pathology. Braak and Del Tredici (2014) counter this argument by suggesting that amyloid plaques may develop after neurofibrillary tangle pathology develops in sites associated with AD (e.g., MTL); therefore, the “absence of Aβ deposits is not an adequate rationale for excluding tau-only cases from the developmental spectrum of the AD-related process.” They further argue that requiring a minimum threshold level of amyloid deposition for a neuropathologic diagnosis of AD (as in the PART criteria) may be justified only when applied in cases with clinically evident dementia, but not when applied to non-demented individuals.
As we move toward the end of the current decade, it is clear that the dogma that “amyloidosis is AD” is giving way to a broader conceptualization of the disease. This is evident in the adoption of biomarker staging systems that are agnostic to the temporal order of their occurrence (e.g., a tally system or the A/T/N system), and with the acceptance of new evidence that brainstem tauopathy and its propagation to the MTL may occur before amyloidosis associated with late-onset sporadic AD. This new understanding of AD may drive fundamental shifts in biomarker strategies, drug discovery, and therapeutics.
Neuropsychology has played a critical role in characterizing the cognitive changes associated with AD and related dementing disorders. This has improved the ability to accurately diagnose AD and differentiate it from other dementing disorders, to identify subtle cognitive changes that occur in the preclinical/prodromal phase of disease, and to track progression of the disease over the aging-MCI-AD continuum. Recent advances in AD biomarker development will alter this role. Increasingly, diagnosticians and investigators will be asked to use an array of available biomarkers to identify the neuropathologic determinants underlying cognitive changes within a given individual, and to detect neuropathology in its earliest stages before the onset of significant cognitive change.
That is not to say, however, that neuropsychology will cease to play an important role in dementia assessment and research. Regardless of the underlying pathology, it remains a critical function to identify the onset and nature of the earliest cognitive deficits that might impact someone’s life, to be able to predict the course of cognitive decline, and to measure the cognitive outcome of future treatments. These functions will likely be enhanced by integrating biomarker information into assessments. The use of such a “precision medicine” approach might bring increased specificity to the study of dementia in the future.
It has also become clear that, as age increases, there is increasing heterogeneity in the neuropathology underlying what is clinically diagnosed as “AD dementia.” Nelson and colleagues (2011) showed that the prevalence of AD pathology increases with age but reaches a plateau at approximately age 90; however, the prevalence of dementia and other pathologies, such as cerebrovascular disease or hippocampal sclerosis (arteriolosclerosis more generally), continue to increase with age (see Figure 6 ). This observation suggests that, in some cases, “AD dementia” appears only following the addition of other pathologies to a sub-threshold level of AD pathology. Such pathological heterogeneity leads to neuropsychological heterogeneity, making dementia characterization and differential diagnosis more difficult.
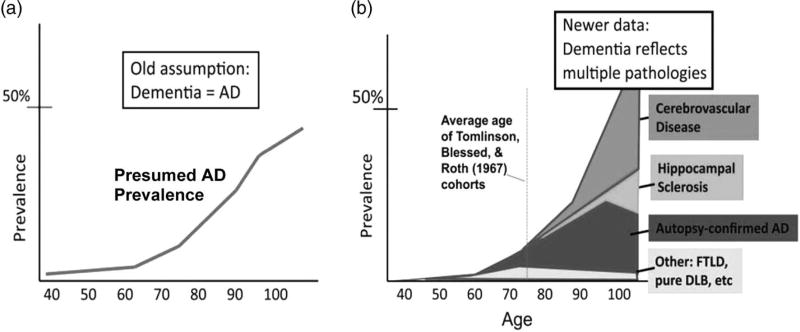
Nelson et al.’s (2011) contrasting depictions of the epidemiology of dementia. Panel (a) is the schematic representation of the prevailing view of Alzheimer neuropathology by age, whereas panel (b) depicts distinct brain diseases other than AD that may contribute to cognitive impairment in late life (adapted from Nelson et al., 2011 ).
In the future, a precision medicine approach will allow multiple biomarkers to target distinct pathologies to show which pathologies are present, a genetic analysis will allow polygenic risk for various disorders to be assessed, and neuropsychological assessment will identify distinct patterns of deficits that reflect the differential impact of distinct pathologies on the dementia syndrome. Movement toward this goal is illustrated in a recent study which showed that individuals diagnosed as amnestic MCI in the ADNI cohort had great heterogeneity in the pattern of cognitive deficits they exhibited and that their deficits coincided well with specific regions of cortical thinning on neuroimaging (see Figure 7 ; Edmonds et al., 2016 ). These results demonstrate the potential utility of a combination of neuropsychological assessment and neuroimaging biomarkers to help explain a heterogeneous presentation of prodromal AD.
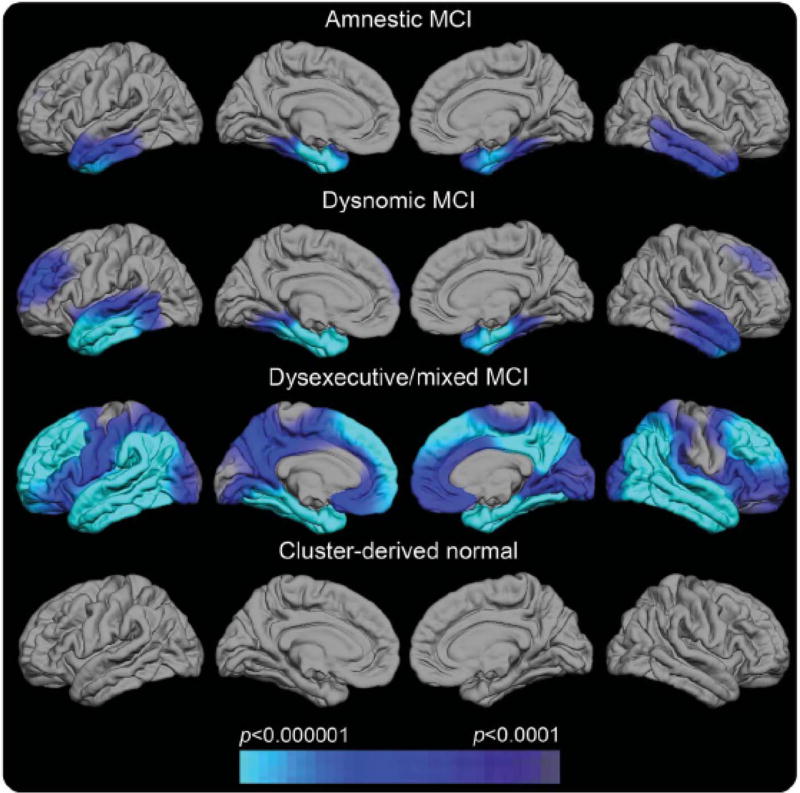
Regional cortical thickness maps of the left and right lateral and medial pial surfaces for each neuropsychological MCI subtype relative to normal control (NC) participants ( Edmonds et al., 2016 ). The scale indicates group differences in cortical thickness at p < .0001. The cyan/blue shades represent areas where the MCI subgroup has significantly thinner cortex than the NC group. Cluster-derived normal (CDN) = those participants who performed normally across the neuropsychological tests but whom ADNI diagnosed as MCI. Their maps show no areas of cortical thinning relative to the NC group, suggesting they are false-positive diagnostic errors. Our prior work showing the CDN subgroup to have normal CSF AD biomarkers and low progression rates adds to the inference that they received false-positive MCI diagnoses ( Bondi et al., 2014 ).
Neuropathological heterogeneity in AD could also have important implications for future therapeutic approaches to the disease. Given the shift away from the amyloid cascade model toward a more equipotential conceptualization of AD, it is not surprising that the recent singular focus on anti-amyloid treatments has led to disappointing results ( Cummings, Morstorf, & Zhong, 2014 ). In an equipotential model of AD, other aspects of AD related pathology may already exist, continue to develop, and adversely affect cognition even if amyloid pathology is removed. If patients in anti-amyloid trials are positive for significant levels of amyloid, the anti-amyloid agent engages and clears amyloid, yet there is no clinical or cognitive benefit, it is reasonable to presume that pathology other than amyloid needs to be targeted.
Since tau pathology is more firmly associated with clinical and cognitive decline than is amyloid pathology, and may accumulate in susceptible regions earlier than that of amyloid, tau-altering pharmacologic interventions would seem worthwhile. Specific therapeutics may also be needed for other underlying pathologies (e.g., arteriolosclerosis, blood–brain barrier dysfunction, α-synuclein) that could be interacting with abnormal amyloid and tau in older individuals with sporadic “AD dementia.” Such agents could be used in a “precision medicine” context, where aberrant biomarkers coupled with a specific pattern of neuropsychological deficits could specify a particular treatment regimen within a prevention framework. Such a framework would also be accommodative of the specter of multiple biomarker abnormalities occurring concurrently.
Over the past century since Alzheimer’s original publication, we have witnessed an explosion of work in the neuropsychology of dementia, and we have much work yet to complete. To borrow from another prominent psychologist who spoke of his perspective to better understand schizophrenia nearly 2 decades ago, Irving Gottesman (2001) pointedly suggested that no discipline committed to understanding any of the major disorders (insert Alzheimer’s disease in this example) has a monopoly on the amounts of uncertainty that remain for current and future generations of investigators. By joining forces across disciplines and assembling the most certain and important facts, investigators can launch new initiatives not previously imagined. Such an effort will be required to solve the complex puzzle of Alzheimer’s disease.
Acknowledgments
This work was supported by National Institutes of Health grants P50 AG05131 (M.W.B., D.P.S.), R01 AG049810 (M.W.B.) and K24 AG026431 (M.W.B.), and the U.S. Department of Veterans Affairs Clinical Sciences Research and Development Service (Career Development Award-2 1IK2 CX001415-01A1 to E.C.E.). Dr. Salmon serves as a consultant for Bristol-Myers Squibb. Dr. Bondi serves as a consultant for Novartis and Eisai and receives royalties from Oxford University Press.
The other authors report no disclosures.
- Albert ML, Feldman RG, Willis AL. The ‘subcortical dementia’ of progressive supranuclear palsy. Journal of Neurology, Neurosurgery, and Psychiatry. 1974;37:121–130. doi: 10.1136/jnnp.37.2.121. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Alzheimer A. über eine eigenartige Erkankung der Hirnrinde. Allgemeine Zeitschrift fur Psychiatrie under Psychisch-Gerichtliche Medizin. 1907;64:146–148. [ Google Scholar ]
- Alzheimer A. Über eigenartige Krankheitsfälle des späteren Alters. Zeitschrift für die Gesamte Neurologie und Psychiatrie. 1911;4:356–385. [ Google Scholar ]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2. Washington, DC: American Psychiatric Association; 1968. [ Google Scholar ]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-III) Washington, DC: American Psychiatric Association; 1980. Task force on nomenclature and statistics. [ Google Scholar ]
- Baddeley AD, Bressi S, Della Sala S, Logie R, Spinnler H. The decline of working memory in Alzheimer’s disease: A longitudinal study. Brain. 1991;114:2521–2542. doi: 10.1093/brain/114.6.2521. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Morris JC Dominantly Inherited Alzheimer Network. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. The New England Journal of Medicine. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bird TD. Clinical genetics of familial Alzheimer’s disease. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer disease. Philadelphia: Lippincott Williams & Wilkens; 1999. pp. 57–66. [ Google Scholar ]
- Blessed G, Tomlinson B, Roth M. The association between quantitative measures of dementia and of senile changes in the cerebral grey matter of elderly subjects. British Journal of Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, Salmon DP. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and prediction of progression. Journal of Alzheimer’s Disease. 2014;42:275–289. doi: 10.3233/JAD-140276. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bondi MW, Monsch AU, Butters N, Salmon DP, Paulsen JS. Utility of a modified version of the Wisconsin Card Sorting Test in the detection of dementia of the Alzheimer type. Clinical Neuropsychologist. 1993;7:161–170. doi: 10.1080/13854049308401518. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bondi MW, Monsch AU, Galasko D, Butters N, Salmon DP, Delis DC. Preclinical cognitive markers of dementia of the Alzheimer’s type. Neuropsychology. 1994;8:374–384. [ Google Scholar ]
- Bondi MW, Salmon DP, Galasko D, Thomas RG, Thal LJ. Neuropsychological function and apolipoprotein E qgenotype in the preclinical detection of Alzheimer’s disease. Psychology and Aging. 1999;14:295–303. doi: 10.1037//0882-7974.14.2.295. [ DOI ] [ PubMed ] [ Google Scholar ]
- Bondi MW, Salmon DP, Monsch AU, Galasko D, Butters N, Klauber MR, Saitoh T. Episodic memory changes are associated with the ApoE-ε4 allele in nondemented older adults. Neurology. 1995;45:2203–2206. doi: 10.1212/wnl.45.12.2203. [ DOI ] [ PubMed ] [ Google Scholar ]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [ DOI ] [ PubMed ] [ Google Scholar ]
- Braak H, Del Tredici K. Are cases with tau pathology occurring in the absence of Aβ deposits part of the AD-related pathological process? Acta Neuropathologica. 2014;128:767–772. doi: 10.1007/s00401-014-1356-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- Braak H, Del Tredici K. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain. 2015;138:2814–2833. doi: 10.1093/brain/awv236. [ DOI ] [ PubMed ] [ Google Scholar ]
- Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. Journal of Neuropathology & Experimental Neurology. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [ DOI ] [ PubMed ] [ Google Scholar ]
- Braak H, Zetterberg H, Del Tredici K, Blennow K. Intraneuronal tau aggregation precedes diffuse plaque deposition, but amyloid-β changes occur before increases of tau in cerebrospinal fluid. Acta Neuropathologica. 2013;126:631–641. doi: 10.1007/s00401-013-1139-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- Brosch JR, Farlow MR, Risacher SL, Apostolova LG. Tau imaging in Alzheimer’s disease diagnosis and clinical trials. Neurotherapeutics. 2017;14:62–68. doi: 10.1007/s13311-016-0490-y. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Buschke H, Sliwinski MJ, Kuslansky G, Lipton RB. Diagnosis of early dementia by the double memory test. Neurology. 1997;48:989–997. doi: 10.1212/wnl.48.4.989. [ DOI ] [ PubMed ] [ Google Scholar ]
- Butters N, Granholm E, Salmon DP, Grant I, Wolfe J. Episodic and semantic memory: A comparison of amnesic and demented patients. Journal of Clinical and Experimental Neuropsychology. 1987;9:479–497. doi: 10.1080/01688638708410764. [ DOI ] [ PubMed ] [ Google Scholar ]
- Caine D. Posterior cortical atrophy: a review of the literature. Neurocase. 2004;10:382–385. doi: 10.1080/13554790490892239. [ DOI ] [ PubMed ] [ Google Scholar ]
- Chertkow H, Bub D. Semantic memory loss in dementia of Alzheimer’s type. Brain. 1990;113:397–417. doi: 10.1093/brain/113.2.397. [ DOI ] [ PubMed ] [ Google Scholar ]
- Clark LR, Delano-Wood L, Libon DJ, McDonald CR, Nation DA, Bangen KJ, Bondi MW. Are empirically derived subtypes of mild cognitive impairment consistent with conventional subtypes? Journal of the International Neuropsychological Society. 2013;19:635–645. doi: 10.1017/S1355617713000313. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Collette F, Van der Linden M, Bechet S, Salmon E. Phonological loop and central executive functioning in Alzheimer’s disease. Neuropsychologia. 1999;37:905–918. doi: 10.1016/s0028-3932(98)00148-1. [ DOI ] [ PubMed ] [ Google Scholar ]
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Nelson PT. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathologica. 2014;128:755–766. doi: 10.1007/s00401-014-1349-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cronin-Golomb A, Amick M. Spatial abilities in aging, Alzheimer’s disease, and Parkinson’s disease. In: Boller F, Cappa SF, editors. Handbook of Neuropsychology. Aging and dementia. 2. Vol. 6. Amsterdam: Elsevier; 2001. pp. 119–143. [ Google Scholar ]
- Cummings JL. Subcortical dementia. New York: Oxford University Press; 1990. [ Google Scholar ]
- Cummings JL, Benson DF. Dementia: A clinical approach. Boston: Butterworth-Heinemann; 1992. [ Google Scholar ]
- Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimer’s Research & Therapy. 2014;6:37. doi: 10.1186/alzrt269. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Delis DC, Massman PJ, Butters N, Salmon DP, Cermak LS, Kramer JH. Profiles of demented and amnesic patients on the California verbal learning test: Implications for the assessment of memory disorders. Psychological Assessment. 1991;3:19–26. [ Google Scholar ]
- Drachman DA. The amyloid hypothesis, time to move on: Amyloid is the downstream result, not cause, of Alzheimer’s disease. Alzheimer’s & Dementia. 2014;10:372–380. doi: 10.1016/j.jalz.2013.11.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- Edmonds EC, Delano-Wood L, Clark LR, Jak AJ, Nation DA, McDonald CR, Bondi MW. Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimer’s & Dementia. 2015;11:415–424. doi: 10.1016/j.jalz.2014.03.005. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW. Subtle cognitive decline and biomarker staging in preclinical Alzheimer’s disease. Journal of Alzheimer’s Disease. 2015;47:231–242. doi: 10.3233/JAD-150128. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Edmonds EC, Eppig J, Bondi MW, Leyden KM, Goodwin B, Delano-Wood L, McDonald CR. Heterogeneous cortical atrophy patterns not captured by conventional diagnostic criteria. Neurology. 2016;87:2108–2116. doi: 10.1212/WNL.0000000000003326. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Folstein MF, Folstein SE, McHugh PR. “Minimental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [ DOI ] [ PubMed ] [ Google Scholar ]
- Frisoni GB, Fox NC, Jack CR, Jr, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer’s disease. Nature Reviews Neurology. 2010;6:67–77. doi: 10.1038/nrneurol.2009.215. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gomar JJ, Conejero-Goldberg C, Davies P, Goldberg TE Alzheimer’s Disease Neuroimaging Initiative. Extension and refinement of the predictive value of different classes of markers in ADNI: Four-year follow-up data. Alzheimer’s & Dementia. 2014;10:704–712. doi: 10.1016/j.jalz.2013.11.009. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55:335–346. doi: 10.1002/ana.10825. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gottesman II. Psychopathology through a life span-genetic prism. American Psychologist. 2001;56:867–878. doi: 10.1037/0003-066x.56.11.867. [ DOI ] [ PubMed ] [ Google Scholar ]
- Heister D, Brewer JB, Magda S, Blennow K, McEvoy LK Alzheimer’s Disease Neuroimaging Initiative. Predicting MCI outcome with clinically available MRI and CSF biomarkers. Neurology. 2011;77:1619–1628. doi: 10.1212/WNL.0b013e3182343314. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hodges JR, Patterson K. Is semantic memory consistently impaired early in the course of Alzheimer’s disease? Neuroanatomical and diagnostic implications. Neuropsychologia. 1995;33:441–459. doi: 10.1016/0028-3932(94)00127-b. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hodges JR, Salmon DP, Butters N. Semantic memory impairment in Alzheimer’s disease: Failure of access or degraded knowledge? Neuropsychologia. 1992;30:301–314. doi: 10.1016/0028-3932(92)90104-t. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hof PR, Vogt BA, Bouras C, Morrison JH. Atypical form of Alzheimer’s disease with prominent posterior cortical atrophy: A review of lesion distribution and circuit disconnection in cortical visual pathways. Vision Research. 1997;37:3609–3625. doi: 10.1016/S0042-6989(96)00240-4. [ DOI ] [ PubMed ] [ Google Scholar ]
- Huber SJ, Shuttleworth EC, Paulson GW, Bellchambers MJ, Clapp LE. Cortical vs subcortical dementia. Neuropsychological differences. Archives of Neurology. 1986;43:392–394. doi: 10.1001/archneur.1986.00520040072023. [ DOI ] [ PubMed ] [ Google Scholar ]
- Hyman BT, Damasio AR, Van Hoesen GW, Barnes CL. Alzheimer’s disease: cell specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [ DOI ] [ PubMed ] [ Google Scholar ]
- Iba M, McBride JD, Guo JL, Zhang B, Trojanowski JQ, Lee VMY. Tau pathology spread in PS19 tau transgenic mice following locus coeruleus (LC) injections of synthetic tau fibrils is determined by the LC’s afferent and efferent connections. Acta Neuropathologica. 2015;130:349–362. doi: 10.1007/s00401-015-1458-4. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, Dubois B. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–347. doi: 10.1212/WNL.0000000000002923. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jack CR, Jr, Knopman DS, Chetelat G, Dickson D, Fagan AM, Frisoni GB, Vos SJB. Suspected non-Alzheimer disease pathophysiology – Concept and controversy. Nature Reviews Neurology. 2016;12:117–124. doi: 10.1038/nrneurol.2015.251. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Trojanowski JQ. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurology. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurology. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jacobs D, Salmon DP, Tröster AI, Butters N. Intrusion errors in the figural memory of patients with Alzheimer’s and Huntington’s disease. Archives of Clinical Neuropsychology. 1990;5:49–57. [ PubMed ] [ Google Scholar ]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC. Quantification of five neuropsychological approaches to defining mild cognitive impairment. American Journal of Geriatric Psychiatry. 2009;17:368–375. doi: 10.1097/JGP.0b013e31819431d5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jedynak BM, Lang A, Liu B, Katz E, Zhang Y, Wyman BT Alzheimer’s Disease Neuroimaging Initiative. A computational neurodegenerative disease progression score: Method and results with the ADNI cohort. NeuroImage. 2012;63:1478–1486. doi: 10.1016/j.neuroimage.2012.07.059. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G Subjective Cognitive Decline Initiative (SCD-I) Working Group. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia. 2014;10:844–852. doi: 10.1016/j.jalz.2014.01.001. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Johnson JK, Head E, Kim R, Starr A, Cotman CW. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Archives of Neurology. 1999;56:1233–1239. doi: 10.1001/archneur.56.10.1233. [ DOI ] [ PubMed ] [ Google Scholar ]
- Katzman R. The prevalence and malignancy of Alzheimer disease: A major killer. Archives of Neurology. 1976;33:217–218. doi: 10.1001/archneur.1976.00500040001001. [ DOI ] [ PubMed ] [ Google Scholar ]
- Katzman R, Kawas C. The epidemiology of dementia and Alzheimer disease. In: Terry RD, Katzman R, Bick KL, editors. Alzheimer disease. New York: Raven Press; 1994. pp. 105–122. [ Google Scholar ]
- Knopman DS, Jack CR, Jr, Wiste HJ, Weigand SD, Vemuri P, Lowe VJ, Petersen RC. Brain injury biomarkers are not dependent on β-amyloid in normal elderly. Annals of Neurology. 2013;73:472–480. doi: 10.1002/ana.23816. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kraepelin E. Psychiatrie: Ein Lehrbuch fur studierende und artzte. In: Kraepelin E, editor. Handbook of psychiatry. 8. Leipzig: Barth; 1910. pp. 593–632. [ Google Scholar ]
- Lefleche G, Albert MS. Executive function deficits in mild Alzheimer’s disease. Neuropsychology. 1995;9:313–320. [ Google Scholar ]
- Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS Alzheimer’s Disease Neuroimaging Initiative. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- La Rue A, Matsuyama SS, McPherson S, Sherman J, Jarvik LF. Cognitive performance in relatives of patients with probable Alzheimer disease: An age at onset effect? Journal of Clinical and Experimental Neuropsychology. 1992;14:533–538. doi: 10.1080/01688639208402842. [ DOI ] [ PubMed ] [ Google Scholar ]
- Mahandra B. Dementia: A survey of the syndrome of dementia. Lancaster, England: MTP; 1984. [ Google Scholar ]
- Mathis CA, Wang Y, Holt DP, Huang G-F, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. Journal of Medicinal Chemistry. 2003;46:2740–2754. doi: 10.1021/jm030026b. [ DOI ] [ PubMed ] [ Google Scholar ]
- Maurer K, Maurer U. Alzheimer: The life of a physician and career of a disease. New York: Columbia University Press; 2003. [ Google Scholar ]
- McHugh PR, Folstein MF. Psychiatric symptoms of Huntington’s chorea: A clinical and phenomenologic study. In: Benson DF, Blumer D, editors. Psychiatric aspects of neurological disease. New York: Raven Press; 1975. pp. 267–285. [ Google Scholar ]
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Kosaka K. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89:88–100. doi: 10.1212/WNL.0000000000004058. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan M. Clinical diagnosis of Alzheimer’s disease: report of the NINCD-ADRDA work group. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [ DOI ] [ PubMed ] [ Google Scholar ]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging – Alzheimer’s Association workgroup. Alzheimer’s & Dementia. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: Clinical characteristics and differences compared to Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2002;14:33–40. doi: 10.1159/000058331. [ DOI ] [ PubMed ] [ Google Scholar ]
- Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, Bigio EH. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Annals of Neurology. 2008;63:709–719. doi: 10.1002/ana.21388. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Miller E. On the nature of the memory disorder in presenile dementia. Neuropsychologia. 1971;9:75–81. doi: 10.1016/0028-3932(71)90064-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- Miller E. Short- and long-term memory in patients with presenile dementia (Alzheimer’s disease) Psychological Medicine. 1973;3:221–224. doi: 10.1017/s003329170004856x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Miller E. Impaired recall and the memory disturbance in presenile dementia. British Journal of Social and Clinical Psychology. 1975;14:73–79. doi: 10.1111/j.2044-8260.1975.tb00151.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Miller E. Retrieval from long-term memory in presenile dementia: two tests of an hypothesis. British Journal of Social and Clinical Psychology. 1978;17:143–148. doi: 10.1111/j.2044-8260.1978.tb00256.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Musiek ES, Holtzman DM. Three dimensions of the amyloid hypothesis: Time, space and ‘wingmen’. Nature Neuroscience. 2015;18:800–806. doi: 10.1038/nn.4018. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nebes R. Semantic memory in Alzheimer’s disease. Psychological Bulletin. 1989;106:377–394. doi: 10.1037/0033-2909.106.3.377. [ DOI ] [ PubMed ] [ Google Scholar ]
- Nelson PT, Head E, Schmitt FA, Davis PR, Neitner JH, Jicha GA, Scheff SW. Alzheimer’s disease is not “brain aging”: Neuropathological, genetic, and epidemiological human studies. Acta Neuropathologica. 2011;121:571–587. doi: 10.1007/s00401-011-0826-y. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nestor PJ, Caine D, Fryer TD, Clarke J, Hodges JR. The topography of metabolic deficits in posterior cortical atrophy (the visual variant of Alzheimer’s disease) with FDG-PET. Journal of Neurology, Neurosurgery, and Psychiatry. 2003;74:1521–1529. doi: 10.1136/jnnp.74.11.1521. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Norton LE, Bondi MW, Salmon DP, Goodglass H. Deterioration of generic knowledge in patients with Alzheimer’s disease: Evidence from the Number Information Test. Journal of Clinical and Experimental Neuropsychology. 1997;19:857–866. doi: 10.1080/01688639708403766. [ DOI ] [ PubMed ] [ Google Scholar ]
- Parasuraman R, Haxby JV. Attention and brain function in Alzheimer’s disease. Neuropsychology. 1993;7:242–272. [ Google Scholar ]
- Perry RJ, Hodges JR. Attention and executive deficits in Alzheimer’s disease: A critical review. Brain. 1999;122:383–404. doi: 10.1093/brain/122.3.383. [ DOI ] [ PubMed ] [ Google Scholar ]
- Petersen RC. Early diagnosis of Alzheimer disease: Is MCI too late? Current Alzheimer Research. 2009;6:324–330. doi: 10.2174/156720509788929237. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Petersen RC, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, Smith GE, Jack CR., Jr Mild cognitive impairment: Ten years later. Archives of Neurology. 2009;66:1447–1455. doi: 10.1001/archneurol.2009.266. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Archives of Neurology. 2005;62:1160–1163. doi: 10.1001/archneur.62.7.1160. [ DOI ] [ PubMed ] [ Google Scholar ]
- Petersen RC, Smith GE, Ivnik RJ, Tangalos EG, Schaid DJ, Thibodeau SN, Kurland LT. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. Journal of the American Medical Association. 1995;273:1274–1278. [ PubMed ] [ Google Scholar ]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [ DOI ] [ PubMed ] [ Google Scholar ]
- Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. New England Journal of Medicine. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [ DOI ] [ PubMed ] [ Google Scholar ]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–2477. doi: 10.1093/brain/awr179. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rascovsky K, Salmon DP, Hansen LA, Thal LJ, Galasko D. Disparate phonemic and semantic fluency deficits in autopsy-confirmed frontotemporal dementia and Alzheimer’s disease. Neuropsychology. 2007;21:20–30. doi: 10.1037/0894-4105.21.1.20. [ DOI ] [ PubMed ] [ Google Scholar ]
- Rathore S, Habes M, Iftikhar MA, Shacklett A, Davatzikos C. A review on neuroimaging-based classification studies and associated feature extraction methods for Alzheimer’s disease and its prodromal stages. NeuroImage. 2017;155:530–548. doi: 10.1016/j.neuroimage.2017.03.057. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Reed T, Carmelli D, Swan GE, Breitner JCS, Welsh KA, Jarvik GP, Auwerx J. Lower cognitive performance in normal older adult male twins carrying the apolipoprotein E ε4 allele. Archives of Neurology. 1994;51:1189–1192. doi: 10.1001/archneur.1994.00540240033012. [ DOI ] [ PubMed ] [ Google Scholar ]
- Renner JA, Burns JM, Hou CE, McKeel DW, Jr, Storandt M, Morris JC. Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology. 2004;63:1175–1180. doi: 10.1212/01.wnl.0000140290.80962.bf. [ DOI ] [ PubMed ] [ Google Scholar ]
- Richard E, Schmand BA, Eikelenboom P, Van Gool WA Alzheimer’s Disease Neuroimaging Initiative. MRI and cerebrospinal fluid biomarkers for predicting progression to Alzheimer’s disease in patients with mild cognitive impairment: a diagnostic accuracy study. BMJ Open. 2013;3:e002541. doi: 10.1136/bmjopen-2012-002541. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rosser A, Hodges JR. Initial letter and semantic category fluency in Alzheimer’s disease, Huntington’s disease, and progressive supranuclear palsy. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57:1389–1394. doi: 10.1136/jnnp.57.11.1389. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rouleau I, Salmon DP, Butters N, Kennedy C, McGuire K. Quantitative and qualitative analyses of clock drawings in Alzheimer’s and Huntington’s disease. Brain and Cognition. 1992;18:70–87. doi: 10.1016/0278-2626(92)90112-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- Ryan NS, Keihaninejad S, Shakespeare TJ, Lehmann M, Crutch SJ, Malone IB, Fox NC. Magnetic resonance imaging evidence for presymptomatic change in thalamus and caudate in familial Alzheimer’s disease. Brain. 2013;136:1399–1414. doi: 10.1093/brain/awt065. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Salmon DP, Bondi MW. Neuropsychological assessment of dementia. Annual Review of Psychology. 2009;60:257–282. doi: 10.1146/annurev.psych.57.102904.190024. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Salmon DP, Heindel WC, Lange KL. Differential decline in word generation from phonemic and semantic categories during the course of Alzheimer’s disease: Implications for the integrity of semantic memory. Journal of the International Neuropsychological Society. 1999;5:692–703. doi: 10.1017/s1355617799577126. [ DOI ] [ PubMed ] [ Google Scholar ]
- Salmon DP, Kwo-on-Yuen PF, Heindel W, Butters N, Thal LJ. Differentiation of Alzheimer’s disease and Huntington’s disease with the Dementia Rating Scale. Archives of Neurology. 1989;46:1204–1208. doi: 10.1001/archneur.1989.00520470060028. [ DOI ] [ PubMed ] [ Google Scholar ]
- Salmon DP, Thomas RG, Pay MM, Booth A, Hofstetter CR, Thal LJ, Kaltzman R. Alzheimer’s disease can be accurately diagnosed in very mildly impaired individuals. Neurology. 2002;59:1022–1028. doi: 10.1212/wnl.59.7.1022. [ DOI ] [ PubMed ] [ Google Scholar ]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D’Angelo G, Mintun MA. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Aβ42. Journal of Neuroscience. 2010;30:17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sims R, Williams J. Defining the genetic architecture of Alzheimer’s disease: Where next? Neurodegenerative Diseases. 2016;16:6–11. doi: 10.1159/000440841. [ DOI ] [ PubMed ] [ Google Scholar ]
- Small BJ, Fratiglioni L, Viitanen M, Winblad B, Bäckman L. The course of cognitive impairment in preclinical Alzheimer disease: three- and six-year follow-up of a population based sample. Archives of Neurology. 2000;57:839–844. doi: 10.1001/archneur.57.6.839. [ DOI ] [ PubMed ] [ Google Scholar ]
- Smith GE, Bondi MW. Mild cognitive impairment and dementia: Definitions, diagnosis, and treatment. New York: Oxford University Press; 2013. [ Google Scholar ]
- Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life. Findings from the Nun Study. Journal of the American Medical Association. 1996;275:528–532. [ PubMed ] [ Google Scholar ]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Squire LR. Memory and brain. New York: Oxford University Press; 1987. [ Google Scholar ]
- Stelzmann RA, Schnitzlein N, Murtagh FR. An English translation of Alzheimer’s paper, “über eine eigenartige Erkankung der Hirnrinde”. Clinical Anatomy. 1995;8:429–431. doi: 10.1002/ca.980080612. [ DOI ] [ PubMed ] [ Google Scholar ]
- Storandt M, Botwinick J, Danziger WL, Berg L, Hughes CP. Psychometric differentiation of mild senile dementia of the Alzheimer type. Archives of Neurology. 1984;41:497–499. doi: 10.1001/archneur.1984.04050170043013. [ DOI ] [ PubMed ] [ Google Scholar ]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein-E – High-avidity binding to B-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:9649–9653. doi: 10.1073/pnas.90.5.1977. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tenovuo O, Kemppainen N, Aalto S, Nagren K, Rinne JO. Posterior cortical atrophy: A rare form of dementia with in vivo evidence of amyloid-beta accumulation. Journal of Alzheimer’s Disease. 2008;15:351–355. doi: 10.3233/jad-2008-15301. [ DOI ] [ PubMed ] [ Google Scholar ]
- Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, Trojanowski JQ. The Alzheimer’s Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimer’s & Dementia. 2013;9:e111–e194. doi: 10.1016/j.jalz.2013.05.1769. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, Petersen RC. Mild cognitive impairment–beyond controversies, towards a consensus: Report of the Inter-national Working Group on Mild Cognitive Impairment. Journal of Internal Medicine. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [ DOI ] [ PubMed ] [ Google Scholar ]
- Wirth M, Madison CM, Rabinovici GD, Oh H, Landau SM, Jagust WJ. Alzheimer’s disease neurodegenerative biomarkers are associated with decreased cognitive function but not β-Amyloid in cognitively normal older individuals. Journal of Neuroscience. 2013;33:5553–5563. doi: 10.1523/JNEUROSCI.4409-12.2013. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- World Health Organization. International Classification of Diseases. 10. Geneva, Switzerland: WHO; 1992. [ Google Scholar ]
- View on publisher site
- PDF (3.4 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO
COMMENTS
Essay Example: Alzheimer's disease, a specific type of dementia, is a complex and devastating condition that impairs cognitive functioning and behavioral abilities, significantly affecting daily life. ... This research paper will delve into Alzheimer's Disease, covering its causes, symptoms, progression, and current treatments. ...
Alzheimer's disease (AD) (named after the German psychiatric Alois Alzheimer) is the most common type of dementia and can be defined as a slowly progressive neurodegenerative disease characterized by neuritic plaques and neurofibrillary tangles as a result of amyloid-beta peptide's (Aβ) accumulation in the most affected area of the brain ...
accounting for up to 70% of all dementia cases in the United States.7 Other types of primary dementia include vascular dementia (10 20%), dementia associated with Parkinson's disease, dementia with Lewy bodies, and frontotemporal dementia. Epidemiology of AD AD is a critical public health issue in the United States
Searching for Alzheimer's disease essay examples? 🙋 Need some titles on memory loss? ☝️ Not an issue! Check our Alzheimer's disease research topics & samples. ... Research Paper Keyword Generator. Automatic Notes Generator.
Introduction. Alzheimer's disease is a progressive neurodegenerative disease that leads to memory deficits and eventually fatality. Around the world, one person is diagnosed with Alzheimer's every 2 seconds currently affecting around 40 million people worldwide but by 2050 it will be 150 million people.
Alzheimer's Disease (AD) is a progressive neurodegenerative disease that results in the loss of memory, motor function, ability to think, and other basic functions required for day-to-day life.
Introduction. Alzheimer`s Disease is a chronic neurodegenerative disorder that is currently affecting an estimated 25 million people across the globe (Burock et al, 2014).
Support your arguments with evidence from Alzheimer's disease research paper works and reputable sources. Remember to cite any sources used in your essay and consider including a list of free essays on Alzheimer's disease or additional resources for readers seeking further information. ... This statement should present a specific viewpoint ...
Alzheimer described Auguste Deter's symptomatology in a paper for the "37 th Meeting of South-West German Psychiatrists in Tubingen", ... research on Alzheimer's disease is focused on finding ways to prevent the formation of beta-amyloid plaques and neurofibrillary tangles as a form of prevention. ... Format: Add to Collections. Create a new ...
Suddenly, AD dementia went from a relatively rare condition to a major public health issue. This led to greater attention to the disease by the public and at the National Institutes of Health, which established the National Alzheimer's Disease Research Center program to study the cause, neuropathology, and clinical characteristics of AD.