
UCL Research Ethics

Apply to UCL's Research Ethics Committees

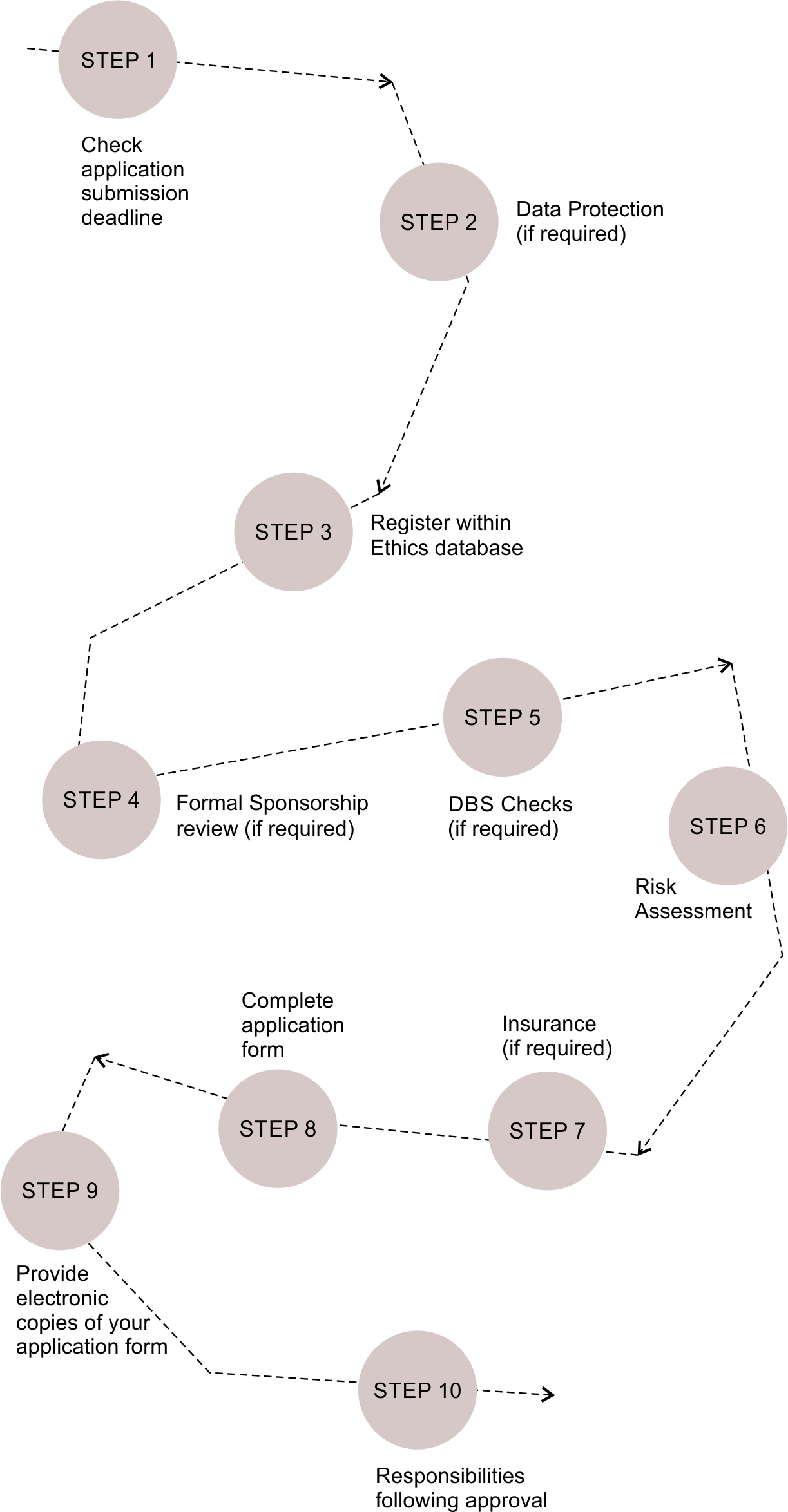
Understand the steps you need to take when submitting high and low-risk applications to the UCL Research Ethics Service
Application routes
You can submit research ethics applications for review through UCL's high- and low-risk routes:
High-risk applications
From 21 October 2024, all high-risk research ethics applications should be submitted via the ethics@ucl system. If you are submitting a high-risk application for the Wednesday 23 October 2024 deadline, you should register your application in our online database and complete the Word application form. All applications for the Wednesday 27 November 2024 deadline need to be submitted via ethics@ucl. Institute of Education (IOE) applicants should contact the IOE Research Ethics Office at [email protected] in the first instance
Low-risk applications
Low-risk research ethics applications submitted centrally to the RIS Research Ethics team need to be sent via the ethics@ucl system. The team will continue to accept low-risk applications from undergraduate and postgraduate taught students via the Word application form until Friday 29 November 2024. Staff and PhD applicants should apply online through ethics@ucl, and Word form applications are no longer accepted. Applicants based in faculties or departments with Local Research Ethics Committees (LRECs), should submit their application as follows:
- LRECs moving to the ethics@ucl system in Phase 1 should indicate which route applicants should use. Phase 1 LRECs are the Institute of Risk and Disaster Reduction; School of Sustainable Construction; Psychology and Language Sciences; Civil, Environmental and Geomatic Engineering; and School of Management
- Applications via all other LRECs should continue using existing local review processes
Applications submitted by email
Applications submitted before the roll-out of ethics@ucl will continue to be processed outside of the system. As per your approval letter, existing approvals should continue to submit amendments and details of adverse events to [email protected] or your local committee . Visit our responsibilities after approval page for more information.
Application process
The steps below detail how to submit high- and low-risk applications to the RIS Research Ethics team. If your department or faculty has established an LREC to review low-risk applications, check local arrangements before starting an application.
If you cannot submit your application through ethics@ucl, contact us at [email protected] .
High-risk projects
Applications involving the following will be deemed high-risk:
- Vulnerable participants
- Sensitivity
- Consent, deception, or covert research
- Disclosures
- Security sensitive materials
- Risk to researcher wellbeing
- Administration of substances
- Invasive procedures
Read our high-risk checklist for more information about these risk categories and examples.
If your project is high-risk, you will need to complete and submit your application in the ethics@ucl system. Yo ur application will be sent to a REC meeting for review. The application deadline and meeting dates can be found below.
Low-risk projects
Applications for research projects not involving any of the above high-risk elements can be reviewed as 'low-risk'. Low-risk applications submitted to the RIS Research Ethics team will be reviewed on a rolling basis and there are no fixed submission deadlines.
Several departments and faculties have established LRECs to review and approve low-risk applications. You should check the arrangements for review within your area before submitting an ethics application.
Research involving the processing of personal data (i.e., information that relates to a directly or indirectly identifiable individual) will need to be registered with the UCL Data Protection Office (DPO) before it is submitted for review. If the DPO advises you to amend your data collection and storage arrangements, this should be updated in your ethics application. UCL staff and students can register their research with the DPO using the online registration form on the DPO website. If you are unable to access or use this form, email [email protected] . After reviewing your application, the DPO will issue a data protection registration number that you should include in your ethics application. Remember to quote your data protection registration number in the appropriate section of your ethics application form as evidence that the project has been registered with the DPO. The data protection registration number is different to your ethics application number. If the Data Protection registration process is taking longer than the advertised 10 days, please submit your ethics application form, marking the sections relating to data protection registration as ‘to follow’ or ‘registration in progress’. Once available, please supply the Research Ethics Service with your data protection registration number alongside any changes the DPO advised you to make to data collection and storage arrangements. Data collection cannot commence until you have ethical approval and your research is registered with the UCL DPO (if required).
To determine any risks associated with your research, i.e., risks to yourself as the researcher and those you are researching, you will need to carry out a risk assessment.
It is a legal requirement that all research is assessed for risk. Refer to your department's local arrangements for risk assessments and UCL Safety Services guidance on how to carry out a risk assessment. The guidance includes how to record a risk assessment and who should approve it.
For further advice contact the UCL Safety Services team at [email protected] .
To access the applicant portal, visit ethics.ucl.ac.uk and log in using your UCL Single Sign-on details. A Desktop@UCL or VPN connection is not required. Supervisors should access the system to view and sign student forms.
If you are a new user, we recommend visiting our Using ethics@ucl page before using the system.
Should you have any questions about ethics@ucl not covered by the guidance above, e-mail [email protected] or join one of our drop-in sessions .
The dynamic application form consists of tailored questions based on your responses. The full form will only be visible once sections A and B have been completed and follow-up questions will appear as you progress through the form. When completing the form, please be aware that responses may be lost if you change an answer to an initial question.
Here are some tips to help you navigate the form and submission process:
- Select 'Introduction and Instructions’ to start your application
- Select the information icons on the right-hand side of each question for guidance, tips, and links to useful resources
- Most questions are mandatory and need to be completed before you can submit the form. Optional questions will be highlighted
- Access templates and frequently asked questions under the 'Help' tab in the top navigation menu
- To return to the overview screen, click ‘Navigate’ in the left-hand-side menu
- Share the application with your collaborator(s) or supervisor by selecting ‘Share’ in the left-hand-side menu. Enter their UCL email address and grant full access to the application
- Share the form with external collaborators by selecting ‘View as PDF’ in the left-hand-side menu menu and saving the form as a PDF document. Only questions that you have already answered will be included
- You can save the application and return to it at any time. Your progress will be automatically saved when you select the 'Next page' button
- Once you have submitted your application, you will not be able to make further changes unless requested by a reviewer or supervisor.
To enable review, your application needs to include a full description of planned activities, your recruitment approach and consideration of potential ethical issues, and supporting documentation (such as participant information sheets, consent forms, interview questions, and survey tools). Incomplete applications will be returned with a request to revise and re-submit.
Recruitment documents for participants
All studies involving the recruitment of participants will use documents such as information sheets and consent forms. For guidance, visit our Producing participant recruitment documents page and read the ‘annotated’ template example forms below:
Template Consent Form
Template Patient Information Leaflet
Formal sponsorship review for clinical trials conducted in developing countries.
Interventional studies in developing countries, i.e., drug, device, or surgery trials, need to be registered in UCL's Interventional Clinical Trials Portal . You need to complete an Entry Questionnaire (EQ) and email this to [email protected] , copying in [email protected] .
Once received, the UCL Institute of Clinical Trials and Methodology (ICTM) Portal Review Group will discuss your EQ and send information next steps. Questions regarding the portal should be sent to [email protected] .
Disclosure and Barring Service (DBS) checks
Research conducted in England and Wales requires a criminal record check if it includes working in 'Regulated' activity with vulnerable groups as defined by the Safeguarding Vulnerable Groups Act 2006 or in a position of trust as defined by the Rehabilitation of Offenders Act Exception Order 1975. Further information and advice are available on the Disclosure and Barring Service (DBS) applications page on the UCL Students website.
Researchers need to take action promptly as it can take a month or more to arrange and complete the check. Clearance is required before entering a position of trust. Please quote your DBS Disclosure Number in section A2 of the application form.
Current students or those who have applied for a place on a programme at UCL that needs DBS clearance should visit the UCL Student Centre to have their DBS form checked and authorised. Students should email [email protected] for information about this in the first instance.
Staff who need a DBS check as part of their work (e.g., for a research project) should contact the Employment Contracts Administration of Human Resources at [email protected] to request a form to complete to decide what level of certification is required. Once a decision has been made, you will need to visit HR in person to collect the DBS form.
As per the UCL DBS Checks and Criminal Convictions Policy , "UCL will accept portability of DBS checks, which individuals may have from previous employers, as proof of satisfactory clearance" under certain conditions. See Section 3.15 of the Policy for details on these conditions.
DBS checks are not applicable for research conducted outside England and Wales (e.g., overseas or in Scotland/Northern Ireland). Students conducting research outside of England and Wales should investigate obtaining a police check for the relevant country.
The GOV.UK website provides information on how to obtain different police checks. Whilst it is not always possible in some countries, it is best practice for students to make every effort to obtain them. Further information about Scottish police checks is available on mygov.scot and Northern Irish police checks on nidirect.gov.
- Criminal records checks for overseas applicants (GOV.UK)
- Disclosure and criminal record checks (mygov.scot)
- Access NI: Criminal Records Checks (nidirect.gov)
For advice on whether you require a DBS, student researchers should email [email protected] . Staff researchers should email [email protected] .
UCL studies are insured through a commercial insurer. For most studies the cover is automatic, however, staff or students undertaking the following types of studies will need to complete an insurance form:
- Intervention studies which enrol over 5,000 subjects
- All clinical intervention studies which enrol children aged 5 years and under where the aim is prophylaxis
- All clinical intervention studies where an inclusion criterion is pregnancy
- All intervention or clinical research studies conducted in the USA and Canada.
Visit the UCLH/UCL Joint Research Office website for further information.

Travel insurance arrangements for students conducting research overseas
Students conducting overseas research need to complete an application form and be issued an insurance cover note. Information and guidance on Travel on UCL business can be found on the UCL Finance website.
At the end of the application, a completeness check will appear highlighting any unanswered mandatory questions. These questions will need to be completed before the form can be signed and submitted.
Applicants will need to complete a declaration confirming they have read and understood the requirements before submitting the form. Additionally, a signature will be required before submission. This will vary depending on the type of application and the associated risks:
Once your application has been reviewed, you will receive an email with reviewer feedback and details about the next steps. Your application will be unlocked, allowing you to make edits based on the feedback, and to re-sign and resubmit your form.
Visit our Guidance, training and resources pages for guidance on responding to reviewer comments. materials for guidance on how to respond to review comments.
The Principal Investigator is expected to report any proposed changes or adverse events, and if required, report on progress at least annually. Visit our responsibilities after approval page for more information.
Application deadlines and meeting dates
There are no research ethics committee meetings in December or August. Please keep this in mind when planning your research. New high-risk applications received after the November and July meeting deadlines will not be reviewed until January and September respectively.
WINTER 2024 LOW-RISK DEADLINE: If you wish to receive initial feedback on a new low-risk ethics application before the Christmas 2024 closure, please submit your application no later than Friday 29 November 2024 . Applications submitted after this date will receive feedback after UCL reopens in the new year.
2023/24 and 2024/25 UCL LMS and HAS REC application deadlines and meeting dates
Page last updated: November 2024

UCL Research Ethics Committee
Procedures for Applications
Check research ethics committee meeting dates and submission deadlines for:.
- Data Protection Registration Submission (if required);
- Ethics Applications for full committee review. Chair's review applications are dealt with on an ad hoc basis so there are no submission deadlines.
Application Deadlines & Meeting Dates →
Data Protection (if required)
If you are proposing to collect personal data i.e. data from which a living individual can be identified you must be registered with the UCL Data Protection Officer before you submit your ethics application for review . If the Data Protection Officer advises you to make changes to the way in which you propose to collect and store the data this should be reflected in your ethics application form.
You can register for Data Protection on the → UCL Legal Services website by downloading the ‘Research Registration Form’. The form should be completed and e-mailed to: [email protected] . The Legal Services should be able to process your application within 10 days upon which time you will be issued with a Data Protection Registration Number.
Please quote your Data Protection registration number in the appropriate section of your application form as evidence that the project has been registered with the UCL Data Protection Office.
Register within the Ethics database and download an ethics application form
- You will need to register your personal (if this is your first application) details on the ethics database ( → Registration Form ) and a personal account will be established for you.
- For existing users log into your account using your email address and password and add a ‘new project’ to your account.
- A unique Project ID for your proposal will be issued immediately which you should make a note of. The finance department will require this Project ID in order to process grant applications based on this research.
- Once you have entered your personal and project details, a zip folder will appear containing a ‘low’ and ‘high’ risk application form together with supporting guidelines for you to download to your computer. You will need to decide based on the criteria of minimal risk e.g. if your research study does not involve vulnerable groups, intrusive interventions [including MRI], sensitive topics and deception, it is likely that a ‘low’ risk application would be appropriate for the REC Chair's review. For studies involving more than minimal risk a ‘high risk’ application form will need to be completed for review by the full ethics committee.
- Low risk applications are reviewed by the REC Chair on an ad hoc basis so you can submit your application at any time, whereas there are application submission deadlines for high risk applications as outlined in Step 1.
Formal Sponsorship Review for Clinical Trials Conducted in Developing Countries (if required)
Clinical trials - drug trials, devices trials and trials in surgery which are conducted in developing countries need the approval of the UCL Research Ethics Committee. Each year the committee reviews a small number of such trials and currently, once ethics approval has been given the investigator can proceed without further review or approval from UCL.
In contrast, clinical trials being conducted in UK or Europe are reviewed by the National Research Ethics Services and require a formal decision from UCL as sponsor of the study. Both the MRC Clinical Trials Unit at UCL and UCL Sponsorship Oversight Committee conduct a rigorous risk assessment of such studies before sponsorship is agreed.
Although sponsorship is currently not a legal requirement for all trials in developing countries, UCL Research Ethics Committee, the Institute Clinical Trials Methodology and Joint Research Office are in agreement that a formal sponsorship review should be introduced for such studies. Not only will this provide parity of review between trials conducted in the UK and Europe and those conducted in developing countries but it will ensure that trials in developing countries of an appropriate standard and that risk mitigation plans are in place.
Disclosure and Barring Service (DBS) Checks (if required)
A criminal record check will be required by law if the research includes working in 'Regulated' activity with vulnerable groups as defined by the Safeguarding Vulnerable Groups Act 2006 or in a position of trust as defined by the Rehabilitation of Offenders Act Exception Order 1975. Further information and advice is available at the → Disclosure and Barring Service (DBS) Checks website. It is imperative that researchers to whom this applies take action promptly. It can take a month or more to arrange and complete the check ; but it is important that researchers have received their clearance before entering a position of trust. Please quote your DBS Disclosure Number in section A2 of the application form. Current students or those who have applied for a place on a programme at UCL which needs DBS clearance must visit the Student Centre to have their DBS form checked and authorised, and should email [email protected] for information about this in the first instance.
Staff who need the DBS check as part of their work (for a research project, for example) should contact the Employment Contracts Administration of Human Resources at [email protected] for a form to complete to decide what level of certificate is required. Once they have seen your completed form and made their decision, you will need to go to HR in person to collect the DBS form itself.
Children: Children should only be involved in research where it is absolutely essential and the information cannot be gained by using an adult participant.
Prisoners: Where a research participant is a prisoner, the permission of the Director of Prisons Health Care is required, in addition to the consent of the participant.
Vulnerable Adults: Research on a participant with a mental health problem or who is temporarily incapacitated is a particularly sensitive area.
Risk Assessment
In order to determine whether there are any risks associated with your research i.e. risks to yourself as the researcher and to those you are researching, it is important to carry out a risk assessment. It is a legal requirement that all research is assessed for risk. Refer to your Departmental arrangements for risk assessment procedures. Alternatively please refer to UCL Safety Services guidance on how to carry out a risk assessment. The guidance includes how to record the assessment which must be authorised by your Supervisor and retained for your records. Please e-mail: [email protected] if you require further advice.
Step 7 (if required)
The insurance for all UCL studies is provided by a commercial insurer. For the majority of studies the cover is automatic. However, staff or students undertaking the following types of studies will need to complete an insurance form (see → UCL Insurance ) when applying to the UCL Research Ethics Committee for ethical approval of their project:
- intervention studies which enrol over 5,000 subjects
- all clinical intervention studies which enrol children aged 5 years and under where the aim is prophylaxis
- all clinical intervention studies where an inclusion criteria is pregnancy
- all intervention or clinical research studies conducted in the USA and Canada
Travel Insurance arrangements for students conducting research overseas an application form will need to be completed so that an insurance cover note can be issued. For further information and guidance please visit → Insurance website .
Complete the application form and appendices
that are applicable to your study ensuring that both the Principal Researcher and the Head of your Department (OR Departmental Ethics Committee Chair/Departmental Ethics Lead) have signed the form.
Please find below sample copies of our ‘low’ and ‘high’ risk application forms and application guidance documents for review purposes only. Please do not attempt to complete these versions.
- Sample Low Risk Application Form
- Sample Guidelines for completing the Low Risk Application Form
- Sample High Risk Application Form
- Sample Guidelines for completing the High Risk Application Form
Recruitment Documents for Participants – All studies that involve the recruitment of participants will use recruitment documents such as information sheets and consent forms. See our ‘annotated’ template example forms: Template Consent Form and Template Participant Information Sheet . Also, see Advice on Formulating Participant Information Sheets .
When the application is complete, please submit an authorised electronic copy of your application to: [email protected] .
It is important that your application is submitted as a single pdf document which contains the electronic signatures of both the Principal Researcher and Head of Department and includes any supporting documentation, all in one file . Please do not provide a scanned physically signed version as this creates a large pdf document.
Following Approval
The Principal Researcher must report any proposed changes, any adverse events and if required report progress on an annual basis (see Key Responsibilities of the Principal Researcher following Approval ).
Page last modified on 15 May 2020

IMAGES