An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Critical Appraisal Toolkit (CAT) for assessing multiple types of evidence
- Author information
- Article notes
- Copyright and License information
Correspondence: [email protected]
Contributor: Jennifer Kruse, Public Health Agency of Canada – Conceptualization and project administration
Series information
Scientific writing
Collection date 2017 Sep 7.
Healthcare professionals are often expected to critically appraise research evidence in order to make recommendations for practice and policy development. Here we describe the Critical Appraisal Toolkit (CAT) currently used by the Public Health Agency of Canada. The CAT consists of: algorithms to identify the type of study design, three separate tools (for appraisal of analytic studies, descriptive studies and literature reviews), additional tools to support the appraisal process, and guidance for summarizing evidence and drawing conclusions about a body of evidence. Although the toolkit was created to assist in the development of national guidelines related to infection prevention and control, clinicians, policy makers and students can use it to guide appraisal of any health-related quantitative research. Participants in a pilot test completed a total of 101 critical appraisals and found that the CAT was user-friendly and helpful in the process of critical appraisal. Feedback from participants of the pilot test of the CAT informed further revisions prior to its release. The CAT adds to the arsenal of available tools and can be especially useful when the best available evidence comes from non-clinical trials and/or studies with weak designs, where other tools may not be easily applied.
Introduction
Healthcare professionals, researchers and policy makers are often involved in the development of public health policies or guidelines. The most valuable guidelines provide a basis for evidence-based practice with recommendations informed by current, high quality, peer-reviewed scientific evidence. To develop such guidelines, the available evidence needs to be critically appraised so that recommendations are based on the "best" evidence. The ability to critically appraise research is, therefore, an essential skill for health professionals serving on policy or guideline development working groups.
Our experience with working groups developing infection prevention and control guidelines was that the review of relevant evidence went smoothly while the critical appraisal of the evidence posed multiple challenges. Three main issues were identified. First, although working group members had strong expertise in infection prevention and control or other areas relevant to the guideline topic, they had varying levels of expertise in research methods and critical appraisal. Second, the critical appraisal tools in use at that time focused largely on analytic studies (such as clinical trials), and lacked definitions of key terms and explanations of the criteria used in the studies. As a result, the use of these tools by working group members did not result in a consistent way of appraising analytic studies nor did the tools provide a means of assessing descriptive studies and literature reviews. Third, working group members wanted guidance on how to progress from assessing individual studies to summarizing and assessing a body of evidence.
To address these issues, a review of existing critical appraisal tools was conducted. We found that the majority of existing tools were design-specific, with considerable variability in intent, criteria appraised and construction of the tools. A systematic review reported that fewer than half of existing tools had guidelines for use of the tool and interpretation of the items ( 1 ). The well-known Grading of Recommendations Assessment, Development and Evaluation (GRADE) rating-of-evidence system and the Cochrane tools for assessing risk of bias were considered for use ( 2 ), ( 3 ). At that time, the guidelines for using these tools were limited, and the tools were focused primarily on randomized controlled trials (RCTs) and non-randomized controlled trials. For feasibility and ethical reasons, clinical trials are rarely available for many common infection prevention and control issues ( 4 ), ( 5 ). For example, there are no intervention studies assessing which practice restrictions, if any, should be placed on healthcare workers who are infected with a blood-borne pathogen. Working group members were concerned that if they used GRADE, all evidence would be rated as very low or as low quality or certainty, and recommendations based on this evidence may be interpreted as unconvincing, even if they were based on the best or only available evidence.
The team decided to develop its own critical appraisal toolkit. So a small working group was convened, led by an epidemiologist with expertise in research, methodology and critical appraisal, with the goal of developing tools to critically appraise studies informing infection prevention and control recommendations. This article provides an overview of the Critical Appraisal Toolkit (CAT). The full document, entitled Infection Prevention and Control Guidelines Critical Appraisal Tool Kit is available online ( 6 ).
Following a review of existing critical appraisal tools, studies informing infection prevention and control guidelines that were in development were reviewed to identify the types of studies that would need to be appraised using the CAT. A preliminary draft of the CAT was used by various guideline development working groups and iterative revisions were made over a two year period. A pilot test of the CAT was then conducted which led to the final version ( 6 ).
The toolkit is set up to guide reviewers through three major phases in the critical appraisal of a body of evidence: appraisal of individual studies; summarizing the results of the appraisals; and appraisal of the body of evidence.
Tools for critically appraising individual studies
The first step in the critical appraisal of an individual study is to identify the study design; this can be surprisingly problematic, since many published research studies are complex. An algorithm was developed to help identify whether a study was an analytic study, a descriptive study or a literature review (see text box for definitions). It is critical to establish the design of the study first, as the criteria for assessment differs depending on the type of study.
Definitions of the types of studies that can be analyzed with the Critical Appraisal Toolkit*
Analytic study: A study designed to identify or measure effects of specific exposures, interventions or risk factors. This design employs the use of an appropriate comparison group to test epidemiologic hypotheses, thus attempting to identify associations or causal relationships.
Descriptive study: A study that describes characteristics of a condition in relation to particular factors or exposure of interest. This design often provides the first important clues about possible determinants of disease and is useful for the formulation of hypotheses that can be subsequently tested using an analytic design.
Literature review: A study that analyzes critical points of a published body of knowledge. This is done through summary, classification and comparison of prior studies. With the exception of meta-analyses, which statistically re-analyze pooled data from several studies, these studies are secondary sources and do not report any new or experimental work.
* Public Health Agency of Canada. Infection Prevention and Control Guidelines Critical Appraisal Tool Kit ( 6 )
Separate algorithms were developed for analytic studies, descriptive studies and literature reviews to help reviewers identify specific designs within those categories. The algorithm below, for example, helps reviewers determine which study design was used within the analytic study category ( Figure 1 ). It is based on key decision points such as number of groups or allocation to group. The legends for the algorithms and supportive tools such as the glossary provide additional detail to further differentiate study designs, such as whether a cohort study was retrospective or prospective.
Figure 1. Algorithm for identifying the type of analytic study.
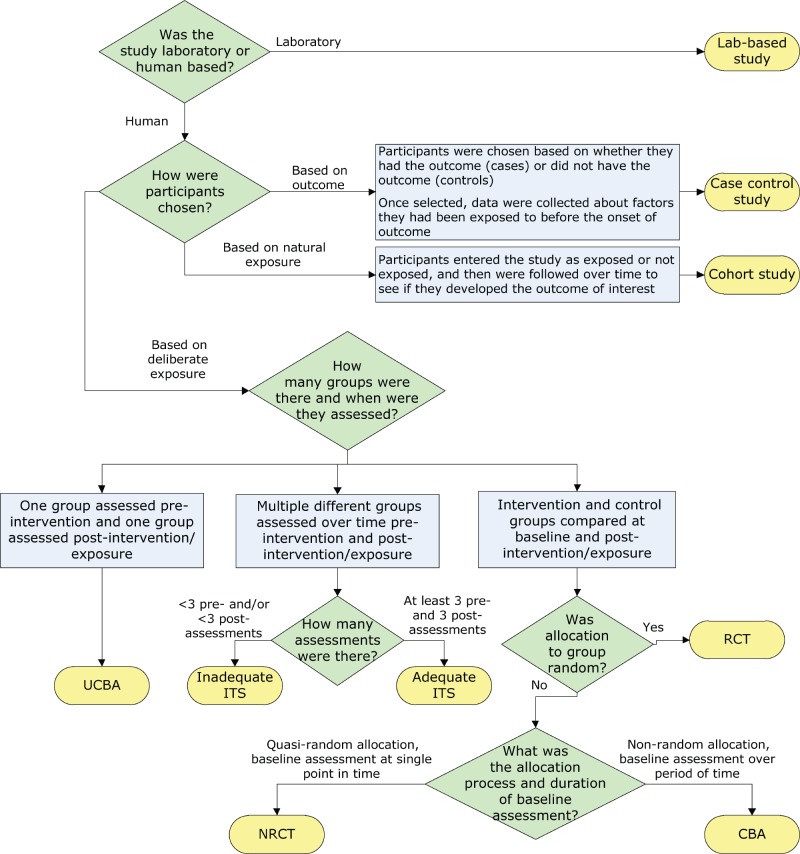
Abbreviations: CBA, controlled before-after; ITS, interrupted time series; NRCT, non-randomized controlled trial; RCT, randomized controlled trial; UCBA, uncontrolled before-after
Separate critical appraisal tools were developed for analytic studies, for descriptive studies and for literature reviews, with relevant criteria in each tool. For example, a summary of the items covered in the analytic study critical appraisal tool is shown in Table 1 . This tool is used to appraise trials, observational studies and laboratory-based experiments. A supportive tool for assessing statistical analysis was also provided that describes common statistical tests used in epidemiologic studies.
Table 1. Aspects appraised in analytic study critical appraisal tool.
The descriptive study critical appraisal tool assesses different aspects of sampling, data collection, statistical analysis, and ethical conduct. It is used to appraise cross-sectional studies, outbreak investigations, case series and case reports.
The literature review critical appraisal tool assesses the methodology, results and applicability of narrative reviews, systematic reviews and meta-analyses.
After appraisal of individual items in each type of study, each critical appraisal tool also contains instructions for drawing a conclusion about the overall quality of the evidence from a study, based on the per-item appraisal. Quality is rated as high, medium or low. While a RCT is a strong study design and a survey is a weak design, it is possible to have a poor quality RCT or a high quality survey. As a result, the quality of evidence from a study is distinguished from the strength of a study design when assessing the quality of the overall body of evidence. A definition of some terms used to evaluate evidence in the CAT is shown in Table 2 .
Table 2. Definition of terms used to evaluate evidence.
* Considered strong design if there are at least two control groups and two intervention groups. Considered moderate design if there is only one control and one intervention group
Tools for summarizing the evidence
The second phase in the critical appraisal process involves summarizing the results of the critical appraisal of individual studies. Reviewers are instructed to complete a template evidence summary table, with key details about each study and its ratings. Studies are listed in descending order of strength in the table. The table simplifies looking across all studies that make up the body of evidence informing a recommendation and allows for easy comparison of participants, sample size, methods, interventions, magnitude and consistency of results, outcome measures and individual study quality as determined by the critical appraisal. These evidence summary tables are reviewed by the working group to determine the rating for the quality of the overall body of evidence and to facilitate development of recommendations based on evidence.
Rating the quality of the overall body of evidence
The third phase in the critical appraisal process is rating the quality of the overall body of evidence. The overall rating depends on the five items summarized in Table 2 : strength of study designs, quality of studies, number of studies, consistency of results and directness of the evidence. The various combinations of these factors lead to an overall rating of the strength of the body of evidence as strong, moderate or weak as summarized in Table 3 .
Table 3. Criteria for rating evidence on which recommendations are based.
A unique aspect of this toolkit is that recommendations are not graded but are formulated based on the graded body of evidence. Actions are either recommended or not recommended; it is the strength of the available evidence that varies, not the strength of the recommendation. The toolkit does highlight, however, the need to re-evaluate new evidence as it becomes available especially when recommendations are based on weak evidence.
Pilot test of the CAT
Of 34 individuals who indicated an interest in completing the pilot test, 17 completed it. Multiple peer-reviewed studies were selected representing analytic studies, descriptive studies and literature reviews. The same studies were assigned to participants with similar content expertise. Each participant was asked to appraise three analytic studies, two descriptive studies and one literature review, using the appropriate critical appraisal tool as identified by the participant. For each study appraised, one critical appraisal tool and the associated tool-specific feedback form were completed. Each participant also completed a single general feedback form. A total of 101 of 102 critical appraisals were conducted and returned, with 81 tool-specific feedback forms and 14 general feedback forms returned.
The majority of participants (>85%) found the flow of each tool was logical and the length acceptable but noted they still had difficulty identifying the study designs ( Table 4 ).
Table 4. Pilot test feedback on user friendliness.
* Number of tool-specific forms returned for total number of critical appraisals conducted
The vast majority of the feedback forms (86–93%) indicated that the different tools facilitated the critical appraisal process. In the assessment of consistency, however, only four of ten analytic studies appraised (40%), had complete agreement on the rating of overall study quality by participants, the other six studies had differences noted as mismatches. Four of the six studies with mismatches were observational studies. The differences were minor. None of the mismatches included a study that was rated as both high and low quality by different participants. Based on the comments provided by participants, most mismatches could likely have been resolved through discussion with peers. Mismatched ratings were not an issue for the descriptive studies and literature reviews. In summary, the pilot test provided useful feedback on different aspects of the toolkit. Revisions were made to address the issues identified from the pilot test and thus strengthen the CAT.
The Infection Prevention and Control Guidelines Critical Appraisal Tool Kit was developed in response to the needs of infection control professionals reviewing literature that generally did not include clinical trial evidence. The toolkit was designed to meet the identified needs for training in critical appraisal with extensive instructions and dictionaries, and tools applicable to all three types of studies (analytic studies, descriptive studies and literature reviews). The toolkit provided a method to progress from assessing individual studies to summarizing and assessing the strength of a body of evidence and assigning a grade. Recommendations are then developed based on the graded body of evidence. This grading system has been used by the Public Health Agency of Canada in the development of recent infection prevention and control guidelines ( 5 ), ( 7 ). The toolkit has also been used for conducting critical appraisal for other purposes, such as addressing a practice problem and serving as an educational tool ( 8 ), ( 9 ).
The CAT has a number of strengths. It is applicable to a wide variety of study designs. The criteria that are assessed allow for a comprehensive appraisal of individual studies and facilitates critical appraisal of a body of evidence. The dictionaries provide reviewers with a common language and criteria for discussion and decision making.
The CAT also has a number of limitations. The tools do not address all study designs (e.g., modelling studies) and the toolkit provides limited information on types of bias. Like the majority of critical appraisal tools ( 10 ), ( 11 ), these tools have not been tested for validity and reliability. Nonetheless, the criteria assessed are those indicated as important in textbooks and in the literature ( 12 ), ( 13 ). The grading scale used in this toolkit does not allow for comparison of evidence grading across organizations or internationally, but most reviewers do not need such comparability. It is more important that strong evidence be rated higher than weak evidence, and that reviewers provide rationales for their conclusions; the toolkit enables them to do so.
Overall, the pilot test reinforced that the CAT can help with critical appraisal training and can increase comfort levels for those with limited experience. Further evaluation of the toolkit could assess the effectiveness of revisions made and test its validity and reliability.
A frequent question regarding this toolkit is how it differs from GRADE as both distinguish stronger evidence from weaker evidence and use similar concepts and terminology. The main differences between GRADE and the CAT are presented in Table 5 . Key differences include the focus of the CAT on rating the quality of individual studies, and the detailed instructions and supporting tools that assist those with limited experience in critical appraisal. When clinical trials and well controlled intervention studies are or become available, GRADE and related tools from Cochrane would be more appropriate ( 2 ), ( 3 ). When descriptive studies are all that is available, the CAT is very useful.
Table 5. Comparison of features of the Critical Appraisal Toolkit (CAT) and GRADE.
Abbreviation: GRADE, Grading of Recommendations Assessment, Development and Evaluation
The Infection Prevention and Control Guidelines Critical Appraisal Tool Kit was developed in response to needs for training in critical appraisal, assessing evidence from a wide variety of research designs, and a method for going from assessing individual studies to characterizing the strength of a body of evidence. Clinician researchers, policy makers and students can use these tools for critical appraisal of studies whether they are trying to develop policies, find a potential solution to a practice problem or critique an article for a journal club. The toolkit adds to the arsenal of critical appraisal tools currently available and is especially useful in assessing evidence from a wide variety of research designs.
Authors’ Statement
DM – Conceptualization, methodology, investigation, data collection and curation and writing – original draft, review and editing
TO – Conceptualization, methodology, investigation, data collection and curation and writing – original draft, review and editing
KD – Conceptualization, review and editing, supervision and project administration

Acknowledgements
We thank the Infection Prevention and Control Expert Working Group of the Public Health Agency of Canada for feedback on the development of the toolkit, Lisa Marie Wasmund for data entry of the pilot test results, Katherine Defalco for review of data and cross-editing of content and technical terminology for the French version of the toolkit, Laurie O’Neil for review and feedback on early versions of the toolkit, Frédéric Bergeron for technical support with the algorithms in the toolkit and the Centre for Communicable Diseases and Infection Control of the Public Health Agency of Canada for review, feedback and ongoing use of the toolkit. We thank Dr. Patricia Huston, Canada Communicable Disease Report Editor-in-Chief, for a thorough review and constructive feedback on the draft manuscript.
Conflict of interest: None.
Funding: This work was supported by the Public Health Agency of Canada.
- 1. Katrak P, Bialocerkowski AE, Massy-Westropp N, Kumar S, Grimmer KA. A systematic review of the content of critical appraisal tools. BMC Med Res Methodol 2004. Sep;4:22. 10.1186/1471-2288-4-22 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. GRADE Working Group. Criteria for applying or using GRADE. www.gradeworkinggroup.org [Accessed July 25, 2017].
- 3. Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. http://handbook.cochrane.org
- 4. Khan AR, Khan S, Zimmerman V, Baddour LM, Tleyjeh IM. Quality and strength of evidence of the Infectious Diseases Society of America clinical practice guidelines. Clin Infect Dis 2010. Nov;51(10):1147–56. 10.1086/656735 [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Public Health Agency of Canada. Routine practices and additional precautions for preventing the transmission of infection in healthcare settings. http://www.phac-aspc.gc.ca/nois-sinp/guide/summary-sommaire/tihs-tims-eng.php [Accessed December 4, 2015].
- 6. Public Health Agency of Canada. Infection Prevention and Control Guidelines Critical Appraisal Tool Kit. http://publications.gc.ca/collections/collection_2014/aspc-phac/HP40-119-2014-eng.pdf [Accessed December 4, 2015].
- 7. Public Health Agency of Canada. Hand hygiene practices in healthcare settings. https://www.canada.ca/en/public-health/services/infectious-diseases/nosocomial-occupational-infections/hand-hygiene-practices-healthcare-settings.html [Accessed December 4, 2015].
- 8. Ha S, Paquette D, Tarasuk J, Dodds J, Gale-Rowe M, Brooks JI et al. A systematic review of HIV testing among Canadian populations. Can J Public Health 2014. Jan;105(1):e53–62. 10.17269/cjph.105.4128 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Stevens LK, Ricketts ED, Bruneau JE. Critical appraisal through a new lens. Nurs Leadersh (Tor Ont) 2014. Jun;27(2):10–3. 10.12927/cjnl.2014.23843 [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Lohr KN. Rating the strength of scientific evidence: relevance for quality improvement programs. Int J Qual Health Care 2004. Feb;16(1):9–18. 10.1093/intqhc/mzh005 [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Crowe M, Sheppard L. A review of critical appraisal tools show they lack rigor: alternative tool structure is proposed. J Clin Epidemiol 2011. Jan;64(1):79–89. 10.1016/j.jclinepi.2010.02.008 [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Young JM, Solomon MJ. How to critically appraise an article. Nat Clin Pract Gastroenterol Hepatol 2009. Feb;6(2):82–91. 10.1038/ncpgasthep1331 [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Polit DF, Beck CT. Nursing Research: Generating and Assessing Evidence for Nursing Practice. 9 th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. Chapter XX, Literature reviews: Finding and critiquing the evidence p. 94-125. [ Google Scholar ]
- View on publisher site
- PDF (348.4 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
- En español – ExME
- Em português – EME
Critical Appraisal: A Checklist
Posted on 6th September 2016 by Robert Will

Critical appraisal of scientific literature is a necessary skill for healthcare students. Students can be overwhelmed by the vastness of search results. Database searching is a skill in itself, but will not be covered in this blog. This blog assumes that you have found a relevant journal article to answer a clinical question. After selecting an article, you must be able to sit with the article and critically appraise it. Critical appraisal of a journal article is a literary and scientific systematic dissection in an attempt to assign merit to the conclusions of an article. Ideally, an article will be able to undergo scrutiny and retain its findings as valid.
The specific questions used to assess validity change slightly with different study designs and article types. However, in an attempt to provide a generalized checklist, no specific subtype of article has been chosen. Rather, the 20 questions below should be used as a quick reference to appraise any journal article. The first four checklist questions should be answered “Yes.” If any of the four questions are answered “no,” then you should return to your search and attempt to find an article that will meet these criteria.
Critical appraisal of…the Introduction
- Does the article attempt to answer the same question as your clinical question?
- Is the article recently published (within 5 years) or is it seminal (i.e. an earlier article but which has strongly influenced later developments)?
- Is the journal peer-reviewed?
- Do the authors present a hypothesis?
Critical appraisal of…the Methods
- Is the study design valid for your question?
- Are both inclusion and exclusion criteria described?
- Is there an attempt to limit bias in the selection of participant groups?
- Are there methodological protocols (i.e. blinding) used to limit other possible bias?
- Do the research methods limit the influence of confounding variables?
- Are the outcome measures valid for the health condition you are researching?
Critical appraisal of…the Results
- Is there a table that describes the subjects’ demographics?
- Are the baseline demographics between groups similar?
- Are the subjects generalizable to your patient?
- Are the statistical tests appropriate for the study design and clinical question?
- Are the results presented within the paper?
- Are the results statistically significant and how large is the difference between groups?
- Is there evidence of significance fishing (i.e. changing statistical tests to ensure significance)?
Critical appraisal of…the Discussion/Conclusion
- Do the authors attempt to contextualise non-significant data in an attempt to portray significance? (e.g. talking about findings which had a trend towards significance as if they were significant).
- Do the authors acknowledge limitations in the article?
- Are there any conflicts of interests noted?
This is by no means a comprehensive checklist of how to critically appraise a scientific journal article. However, by answering the previous 20 questions based on a detailed reading of an article, you can appraise most articles for their merit, and thus determine whether the results are valid. I have attempted to list the questions based on the sections most commonly present in a journal article, starting at the introduction and progressing to the conclusion. I believe some of these items are weighted heavier than others (i.e. methodological questions vs journal reputation). However, without taking this list through rigorous testing, I cannot assign a weight to them. Maybe one day, you will be able to critically appraise my future paper: How Online Checklists Influence Healthcare Students’ Ability to Critically Appraise Journal Articles.
Feature Image by Arek Socha from Pixabay
Robert Will
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
No Comments on Critical Appraisal: A Checklist
Hi Ella, I have found a checklist here for before and after study design: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools and you may also find a checklist from this blog, which has a huge number of tools listed: https://s4be.cochrane.org/blog/2018/01/12/appraising-the-appraisal/
What kind of critical appraisal tool can be used for before and after study design article? Thanks
Hello, I am currently writing a book chapter on critical appraisal skills. This chapter is limited to 1000 words so your simple 20 questions framework would be the perfect format to cite within this text. May I please have your permission to use your checklist with full acknowledgement given to you as author? Many thanks
Thank you Robert, I came across your checklist via the Royal College of Surgeons of England website; https://www.rcseng.ac.uk/library-and-publications/library/blog/dissecting-the-literature-the-importance-of-critical-appraisal/ . I really liked it and I have made reference to it for our students. I really appreciate your checklist and it is still current, thank you.
Hi Kirsten. Thank you so much for letting us know that Robert’s checklist has been used in that article – that’s so good to see. If any of your students have any comments about the blog, then do let us know. If you also note any topics that you would like to see on the website, then we can add this to the list of suggested blogs for students to write about. Thank you again. Emma.
i am really happy with it. thank you very much
A really useful guide for helping you ask questions about the studies you are reviewing BRAVO
Dr.Suryanujella,
Thank you for the comment. I’m glad you find it helpful.
Feel free to use the checklist. S4BE asks that you cite the page when you use it.
I have read your article and found it very useful , crisp with all relevant information.I would like to use it in my presentation with your permission
That’s great thank you very much. I will definitely give that a go.
I find the MEAL writing approach very versatile. You can use it to plan the entire paper and each paragraph within the paper. There are a lot of helpful MEAL resources online. But understanding the acronym can get you started.
M-Main Idea (What are you arguing?) E-Evidence (What does the literature say?) A-Analysis (Why does the literature matter to your argument?) L-Link (Transition to next paragraph or section)
I hope that is somewhat helpful. -Robert
Hi, I am a university student at Portsmouth University, UK. I understand the premise of a critical appraisal however I am unsure how to structure an essay critically appraising a paper. Do you have any pointers to help me get started?
Thank you. I’m glad that you find this helpful.
Very informative & to the point for all medical students
How can I know what is the name of this checklist or tool?
This is a checklist that the author, Robert Will, has designed himself.
Thank you for asking. I am glad you found it helpful. As Emma said, please cite the source when you use it.
Greetings Robert, I am a postgraduate student at QMUL in the UK and I have just read this comprehensive critical appraisal checklist of your. I really appreciate you. if I may ask, can I have it downloaded?
Please feel free to use the information from this blog – if you could please cite the source then that would be much appreciated.
Robert Thank you for your comptrehensive account of critical appraisal. I have just completed a teaching module on critical appraisal as part of a four module Evidence Based Medicine programme for undergraduate Meducal students at RCSI Perdana medical school in Malaysia. If you are agreeable I would like to cite it as a reference in our module.
Anthony, Please feel free to cite my checklist. Thank you for asking. I hope that your students find it helpful. They should also browse around S4BE. There are numerous other helpful articles on this site.
Subscribe to our newsletter
You will receive our monthly newsletter and free access to Trip Premium.
Related Articles

Risk Communication in Public Health
Learn why effective risk communication in public health matters and where you can get started in learning how to better communicate research evidence.

Why was the CONSORT Statement introduced?
The CONSORT statement aims at comprehensive and complete reporting of randomized controlled trials. This blog introduces you to the statement and why it is an important tool in the research world.

Measures of central tendency in clinical research papers: what we should know whilst analysing them
Learn more about the measures of central tendency (mean, mode, median) and how these need to be critically appraised when reading a paper.

IMAGES
VIDEO