- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar

- FREE Experiments
- Kitchen Science
- Climate Change
- Egg Experiments
- Fairy Tale Science
- Edible Science
- Human Health
- Inspirational Women
- Forces and Motion
- Science Fair Projects
- STEM Challenges
- Science Sparks Books
- Contact Science Sparks
- Science Resources for Home and School

Mixtures for Kids
September 2, 2020 By Emma Vanstone 5 Comments
What is a Mixture?
A mixture is a substance in which two or more substances are mixed but not chemically joined together, meaning that a chemical reaction has not taken place.
Mixtures can be easily separated and the substances in the mixture keep their original properties.
Imagine mixing skittles and full size marshmallows, the individual components (skittles and marshmallows) could easily be separated using a filter and each component of the mixture ( skittles and marshmallow ) doesn’t change.
How to make a mixture
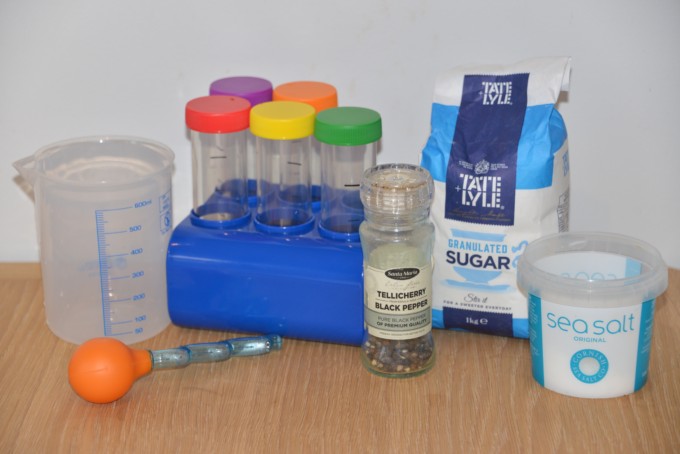
You can make your own mixtures with items from around the house.
1. Firstly try to make a mixture of toys.

2. This time use cereals or sweets.

What is a solution?
A solution is made when a solid (which we call a solute) dissolves into a liquid (that we call the solvent) One example of a solution is salt dissolved in water. The salt and water can be separated again by evaporation ( the water will evaporate if left in a hot place leaving he salt behind ).
Investigation
Aim : To test out these three mixtures to see which form solutions and which don’t
- Salt and Water
- Sugar and Water
- Sand and Water
Results Table
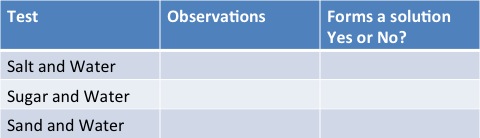
You should find that both salt and water and sugar and water dissolve and form solutions and that sand sinks to bottom!
How do you separate mixtures?
Can you separate the components out of the mixture again? Hint – to separate the sand from water you could use a sieve. This is possible as the sand is insoluble ( doesn’t dissolve in water ).
Salt and sugar are soluble ( dissolve in water ) and can be separated by evaporation.
Another way to separate a mixture is by using a process called chromatography .
Challenge – how would you separate rock salt and water?
Rock salt is a mixture of salt and sand and is often spread on roads in winter to stop cars skidding.
Stage 1 – Grinding
First the rock salt should be ground using a pestle and mortar.
Stage 2- Dissolving
The ground rock salt should be dissolved in a beaker and stirred thoroughly.
Stage 3 – Filtering
The solution of water and rock salt should be passed through the filter paper where the sand ( which will not have dissolved in the water ) will collect.
Salt does dissolve in water and so will pass through the filter paper.
Stage 4 – Evaporating
To separate the salt from the water the water needs to be evaporated off, either by leaving the salty solution in the sunshine or placing under a heat source.
The salt will form as crystals – this process is called crystallisation .

Last Updated on May 24, 2021 by Emma Vanstone
Safety Notice
Science Sparks ( Wild Sparks Enterprises Ltd ) are not liable for the actions of activity of any person who uses the information in this resource or in any of the suggested further resources. Science Sparks assume no liability with regard to injuries or damage to property that may occur as a result of using the information and carrying out the practical activities contained in this resource or in any of the suggested further resources.
These activities are designed to be carried out by children working with a parent, guardian or other appropriate adult. The adult involved is fully responsible for ensuring that the activities are carried out safely.
Reader Interactions
July 18, 2012 at 2:17 pm
Those are great educational play activities. I love your ideas for teaching the difference between a mixture and a solution in a meaningful way. Families can try out your mixtures and solutions and then come up with their own too. Thank you for sharing this on Artsy Play Wednesday on Capri + 3.
: 0 ) Theresa
July 23, 2012 at 6:50 am
Thank you. I’m glad you like it. x
October 08, 2020 at 12:08 pm
This helps me to keep my child busy and I love it So well done!
February 24, 2021 at 8:44 pm
Simple, fun, and accurate!! Thanks from myself and my e-schooled granddaughter 😉
October 08, 2021 at 3:29 pm
I´m a middle school student and and it has helped me understand things better
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *

How to Separate Sugar and Water (Step-By-Step Explanation)
Have you ever thought of separating sugar from water?
I know you might be wondering, is it really possible to remove sugar from water once it’s mixed?
The answer is yes, sugar can be separated from water!
As you may have known, sugar is soluble in water which means that it can be dissolved in water and can form a sugar solution.
Before we go any further, let’s first go through some of the materials you’ll need to gather before beginning this experiment.
What are the materials needed for this experiment?
The materials you would need to complete this experiment can be easily found in any kitchen. These include:
- 1 tablespoon of sugar
- 1 cup of water
- Stove or heat source
Once you have all of your materials, we can move on to the experiment!
How to separate a mixture of sugar and water?
The easiest way or process of separating a mixture of sugar and water involves a three-step procedure: distillation, evaporation, and crystallization.
It’s super easy and we’ll walk you through the step-by-step process to make sure you get it right!
1) Create a mixture of sugar and water.

Mix water and pure sugar in a container. You can directly choose a container that can be directly placed on a heat source. Stir the solution completely to ensure that the sugar is fully dissolved in the water.
2) Heat sugar solution.

Place the container with the sugar solution on a heat source and turn it up to a medium. Wait until boiling water forms bubbles and the mixture becomes hot.
The ideal temperature for separating sugar and water is the boiling point of water, which is 100 degrees Celsius or 212 degrees.
This process is known as distillation which allows the separation of pure solute from the solvent.
One thing to watch out for is to not let the solution boil for too long, especially in high heat, as this can cause the sugar to burn.
3) Wait until the water has completely evaporated.

When a solution is heated for some time, the evaporation process will start and water will rise. The vapors will be collected in the form of steam and the small particles of sugar will be left behind.
Continue to dissolve the water until the pot has almost dried up.
You’ll know when the process is complete when you see only sugar crystals at the bottom of your container.
This process is called crystallization and is how sugar is typically made!
4) Remove the container from the heat source and let it cool.

Once all of the water has evaporated, remove the container from the heat source and allow it to cool down. Once it’s cooled, you can pour out the sugar crystals and enjoy!
And there you have it! You’ve now successfully separated a mixture of sugar and water using the process of distillation.
Here are some questions you might encounter while going through this experiment:
Can I use salt water or seawater for this experiment?
The answer is no. You can only use pure water for this experiment as the salt in sea water or salt water will interfere with the sugar and prevent it from crystallizing.
Can I use any type of sugar for this experiment?
Yes, you can use any type of sugar for this experiment including granulated sugar, brown sugar, or even powdered sugar.
Can I use any type of container for this experiment?
The answer is yes! You can use any type of container that can be placed on a heat source including a pot, saucepan, or even a pan.
We hope this answers any questions you might have had about how to separate a mixture of sugar and water.
If you want to know more about distillation and crystallization, we’ve added some more information below to further help you understand these methods.
What is distillation and how does it work?
Distillation is a method of separating the different components or substances from a liquid mixture by selective boiling and condensation . Distillation allows for the purification of a liquid through the removal of impurities.
The process of distillation can be used to separate many different types of mixtures, including those that are composed of two or more liquids, those that are composed of a liquid and a solid, and those that are composed of a liquid and a gas.
Distillation begins with the heating of the mixture to its boiling point. The vapor that is produced from the boiling mixture is then condensed into a separate container, where it can be collected. The condensation of the vapor produces a liquid that is purer than the original mixture.
The distillation process can be used to purify water, separate alcohol from wine, and extract essential oils from plants.
What is crystallization and how does it work?
Crystallization is the process of separating the components or substances from a liquid mixture by selective cooling and precipitation. Crystallization allows for the purification of a liquid through the removal of impurities.
The crystallization process begins with the cooling of the mixture to its freezing point. The solid that is produced from the cooling mixture is then separated from the liquid. The separation of solids from the liquid produces a purer product.
Crystallization can also be used to create solids from a liquid or gas that did not exist in that form previously.
We hope you found this information helpful and that you now have a better understanding of the distillation and crystallization processes!
Do you love fun experiments like these? Check out this STEM activity book to learn about more cool science experiments you can do at home!
Happy experimenting!

Claire is one of the co-founders of SUPA STEM and is passionate about creating resources that see children flourish. Formerly a high-school teacher and now a mum of two, Claire creates our resources with the heart of a parent and the head of a teacher.

Learning through fun .

Join our newsletter and access our free Resource Vault with fun activities & printables.
Where should we send access?
We take your privacy seriously and will never send spam, share or sell your data. You can unsubscribe from emails easily at any time.

Methods of Separation of Mixtures with Examples
- Updated by Scienly
- On September 29, 2024
We know that the most of the natural substances are not chemically pure substances. They are mixtures that contain two or more kinds of substances. We use different methods of separation to get individual components from the mixture.
Separating or extracting different components from a mixture using some physical methods is called separation of mixtures . We often use it to remove unwanted or harmful components and to separate the individual components from the mixtures.
We can separate heterogeneous mixtures into their respective individual components by simple physical methods like hand picking , threshing , winnowing , sieving , filtration that we use in daily life. However, the choice of techniques to separate components of a mixture depends upon the type of mixture and the difference in the physical and chemical properties of the components of the mixture.
This is because the constituents of a mixture differ in many of their physical and chemical properties. The advantage of this difference in their physical properties is taken in the separation of a mixture. For example, every component keeps its own characteristic properties (like melting point, boiling point, solubility, etc.) even in the mixtures.
Based on the difference between its particular properties of different components of mixtures, we separate them into the mixtures. So, let’s understand the different ways of separating components of mixtures.
Different Methods of Separation of Mixtures
Various techniques or methods employed for the separation of mixtures are as follows:
- Sedimentation (Decantation)
Evaporation
- Sublimation
Solvent Extraction
- Crystallisation or Recrystallisation
- Fractional Crystallisation

Distillation
Fractional distillation, centrifugation, gravity separation, magnetic separation.
Let us understand them in more detail one by one.
Sedimentation
Sedimentation is the process or technique in which insoluble heavy particles in a liquid settle down in the lower part of the container. This simple technique applies to a mixture containing one liquid and another heavier, insoluble solid component. Let’s understand it with the help of an example.
Suppose you are making a tea. You have boiled the water in the saucepan and added some tea leaves into the water. Now keep the prepared tea with tea leaves, aside from some time. Then, you see that the tea leaves will settle down in the lower part of the saucepan. This settling down of particles in the lower part of the container is called sedimentation.
Another example of sedimentation is the settling down of mud particles in the water.

Decantation is the process of transferring the clean liquid getting after sedimentation into another container without disturbing settled particles. After settling down tea leaves, you can pour the clear tea (liquor) into a cup. This transfer of the clear tea is nothing but decantation.
Filtration is a technique of separating a liquid from an insoluble solid in the liquid. This common technique is useful when you want to filter solid particles from the liquid. You can do filtration with the help of various filtering agents, like filter-paper or other materials.
For example, when you make coffee using ground coffee beans, you first need to filter it before drinking so that you don’t get bits of ground coffee beans in our drink.
For this, you can use a filter paper to separate the liquid coffee from the coffee grounds. You place a filter paper inside a funnel and pour the coffee mixture into it. The liquid coffee passes through the tiny pores in the filter paper, while the solid coffee grounds are left behind, giving you a smooth cup of coffee without any bits of ground coffee beans, as shown in the below picture.

Another familiar example of separation of mixture by filtration is as:
(a) Common salt (water soluble) containing sand (insoluble solid) is separated by filtration. For separation, the mixture is shaken so as to dissolve common salt. The soluble components pass through the filter paper and the insoluble solid component sand remains on the filter paper.
The sand collected on the filter-paper is taken in a basin and dried by heating. To obtain the soluble component salt from the water, the filtrate is taken into the evaporating dish and heated until the whole of the water is evaporated. Thus, solid common salt is obtained in the dish.
(b) Sugar (soluble in water) and charcoal (insoluble in water) using water as solvent.
Evaporation is the process of separating a non-volatile soluble solid from its mixture in volatile liquid. This process is useful when solid particles do not break down on heating the solution up to the boiling point of the liquid component.
On heating, the volatile liquid evaporates, leaving behind the soluble solid as residue. For example, this method can separate a homogeneous mixture of sugar and water. Take the solution of sugar and water in the evaporating dish. Heat the dish carefully until the entire water in the dish gets evaporated. The whole crystals of sugar will remain as a residue after evaporation in the dish.

Similarly, we can separate salt from the solution of salt and water, sulphur from the solution of sulphur and carbon disulphide.
Separation by Sublimation
Sublimation is a process in which a solid substance directly converts into its vapours when heated and vapour reconverts into the solid on cooling. This process is useful when one component of the mixture undergoes sublimation and the other components are not broken down (or decomposed) by heating.
For example, iodine can be separated from the sand by sublimation. When the iodine is heated, it sublimes leaving behind the sand. The iodine vapours to be on cooling converts back to solid iodine.

Similarly, other mixtures that can be separated by sublimation are as:
This method is used when the solubility of one component of the mixture in a particular solvent (usually a low-boiling organic solvent) is more than other components. If will more, it forms a distinctly separate layer with the other liquid component if present in the mixture.
For example, a mixture of sulphur and sand can be separated with the help of this method. Since sulphur is soluble in carbon disulphide and sand is insoluble. Therefore, when a solution of carbon disulphide passes through the mixture of sand and sulphur, sulphur gets dissolved in it, as shown in the below figure.

Crystallization or Recrystallisation
Crystals are the purest substance, having a definite geometrical shape. The process by which an impure compound converts into its crystal form is called crystallization. It is one of the most commonly used method for the purification of solid organic compounds. This method is based on the difference between the solubilities of pure compound and impure compound in a particular solvent.
When the impure compound dissolves in a suitable solvent, which is slightly soluble at room temperature but more soluble at higher temperature. On heating, the solution gets concentrated into nearly a saturated solution. When the saturated solution cools, crystals of pure substance will separate out, which are removed by the filtration method.
Fractional Crystallization
This method is used to separate a mixture which contains two solid components having different solubilities in the same solvent. For example, a mixture of potassium nitrate (KNO3) and sodium chloride (NaCl) can be separated out with the help of this method. When the aqueous solution of this mixture is heated and subsequently cooled, the more soluble potassium nitrate (KNO3) remains in the solution and the less soluble sodium chloride (NaCl) separate outs by leaving the more soluble and crystallizes out first, as shown in the below figure.
Another example of this separation method is a mixture of potassium chloride and sodium nitrate, in which potassium chloride is less soluble and crystallizes out first.
Distillation is a process in which a liquid converts into its vapour when heated and then condenses vapours again into the same liquid when cooled. Thus, distillation contains both vaporisation and condensation.
Distillation = Vaporisation + Condensation
This method is commonly used to the separation of that mixture, which contains pure liquid components having a large difference in boiling point. The component having lower boiling point vaporises first and its vapours is condensed using water condenser and then collected as shown in the below figure.
The component having higher boiling point remains behind in the distillation flask. Some examples of separation of mixtures by using this method are as:
- Separation of benzene (boiling point 353 K) and toluene (boiling point 384 K).
- Separation of chlorobenzene and bromobenzene.
You can also use this method of separation for separation and recovery of both components of a solid-liquid mixture. For example, this method can separate a homogeneous mixture of sodium chloride (NaCl) and water. On heating water evaporates and condenses back to the pure water on cooling, whereas sodium chloride (NaCl) left behind in the distillation flask.
Thus, sodium chloride is a soluble solid which recovered as a residue in the distillation flask and water is a liquid component that has recovered as the distillate from the mixture of sodium chloride and water.
We use this method of distillation for the separation of a mixture in which components have a small difference in boiling points. In this method, distillation flask is fitted with a large fractionating column having many bulbs as shown in the below figure.
When the flask heats, liquid vaporises and the vapours rise up from the column. The vapours of less volatile component condense back in the flask and the vapours of only more volatile component escape from the top of the fractionating column, condensed, and collected in a receiver.
For example, gasoline, diesel, kerosene, lubricating oil, etc. from crude petroleum can be separated by using fractional distillation method.
We mostly use the fractional distillation method when boiling point of two liquids of the mixture differ to one another by 10K.
Centrifugation is the process of separating suspended insoluble tiny solid particles from a liquid mixture. It is based on the shape, size, and density of the particles. This process is useful when the normal filtration does not work as well. In the centrifugation process, when we spun a liquid mixture, heavier particles move towards bottom and the lighter particles stay at the top and then get separated.
For example, milk is a mixture of water, solid fat, and other components. When we churn milk rapidly, water which is heavier than fat, move towards bottom and the lighter particles stay at the top and then get drained out.
This method applies to separate a mixture when one component of mixture is magnetic in nature and other non-magnetic components. In this method, the powered mixture falls on a belt of magnetic separator as shown in the below figure.
When the mixture passes over the rollers, the magnetic substance falls vertically down while the non-magnetic substance falls a little away. Some mixtures that the magnetic separation method can separate are:
- Separation of iron ore from sand.
- Iron fillings from sulphur.
We use this process for the separation of a mixture having components of different densities. The powdered mixture undergoes with a stream of running water. The lighter components wash away with water, leaving the heavier ones. For example, a separation of gold particles from rocky substances occurs by gravity separation.
In this tutorial, you have known about various methods of separation of mixtures with examples. Hope that you will have understood the basic points of all techniques of separation of mixtures. Thanks for reading!!!
Related Posts

Electromagnetic Spectrum: Definition, Examples
- October 28, 2024

Wave Nature of Electromagnetic Radiation
- October 23, 2024
What are Isotones: Definition, Examples
- October 19, 2024

What are Isobars: Definition, Examples, Difference
- October 5, 2024
Fun Experiments For Separating Mixtures

Chances are that you separate mixtures often. For example, any time you separate laundry or pick a topping off a pizza or drain a batch of freshly cooked pasta, you are separating a mixture. A mixture is a combination of substances that do not react chemically when they are mixed. According to this definition, a solution — such as sugar water — is a mixture just the same as a mixture of sugar and sand.
Fun Filtering
In this experiment you will observe how rudimentary separating techniques are better suited for certain mixtures, while others require slightly higher technology. Mix uncooked rice, kidney beans and flour in a mixing bowl. Spread the mixture onto a large sheet of wax paper, and you'll notice that the beans are easy to see. Pick them out by hand and place them in a cup. Separating the rice from the flour, however, won't be so easy. Prepare a sieve by cutting out a square section of window screen large enough to fit over the bowl. Set the screen over the mouth of the bowl, and affix it with a large rubber band. Gather the wax paper into a funnel shape, and slowly pour the flour and rice mixture onto the screen. The flour will pass through, leaving the rice on top.
Attracting Opposites
Separating a mixture of identical solids can be challenging until you identify a property that differentiates one from the other. Gather a collection of aluminum bolts and steel bolts, and make sure that both sets are identical. Then, mix the bolts thoroughly in a plastic bowl. Lower a bar magnet toward the bolts. The steel bolts are magnetic and will attract to the magnet as it gets close. As space on the magnet fills, remove the attracted bolts and place them in a separate container. Keep passing the magnet over the bowl until you have removed all of the steel bolts.
Skim Pickin's
Mix plastic marbles and glass marbles together in two large bowls. Take one bowl for yourself and give the other bowl to a partner. Tell your partner he or she will have to separate that mixture by hand and that you will separate the mixture using only a cup of water. Predict who will be able to separate the mixture faster. Ready a timer, and fill a large cup with water. Start the timer, and let your partner begin picking out the plastic marbles. Pour the cup of water into your bowl and watch as the plastic marbles immediately float to the surface while the glass marbles remain at the bottom.
Mystery Mixture
Have a friend create a batch of mystery mix for you to separate. Your friend can use any or all of the following ingredients: water, sand, sugar, soil and vegetable oil. When your friend presents the mixture, split it into several samples and perform experiments to identify its individual components. For example, if water is present, you can first try filtering the solution through a coffee filter to remove any sand or dirt. You could then boil the water to reveal the presence of sugar. If the mixture is dry but looks oily, you can add water to make the oil rise to the surface, and then skim it off.
- Science Web: Mixing and Separating
- Frostburg State University: Separating Mixtures
- Science World: Mysterious Mixtures
Cite This Article
Cascio, Christopher. "Fun Experiments For Separating Mixtures" sciencing.com , https://www.sciencing.com/fun-experiments-separating-mixtures-13769/. 9 March 2018.
Cascio, Christopher. (2018, March 9). Fun Experiments For Separating Mixtures. sciencing.com . Retrieved from https://www.sciencing.com/fun-experiments-separating-mixtures-13769/
Cascio, Christopher. Fun Experiments For Separating Mixtures last modified March 24, 2022. https://www.sciencing.com/fun-experiments-separating-mixtures-13769/
Recommended

Classes for Curious minds
A place for children to learn, experiment, explore and play
For children aged 3-12 years
Sugar Water Density Rainbow Experiment
Learn about density in this fun and simple science experiment.
November 2019

You Will Need
- Food colouring
- Pipette/syringe
The Experiment
- Fill 4 glasses with water. Leave the 5th glass empty.
- Add 2-3 drops of food colouring to each glass of water. Add red colouring to the first glass, yellow to the second, green to the third, and blue to the fourth.
- In the glass with red colouring, do not add any sugar. In the yellow glass, add one tablespoon of sugar. In the green glass add two tablespoons of sugar. In the blue glass add three tablespoons of sugar. Leave the fifth glass empty. Stir the sugar/food colouring/water solution in each glass until the sugar is completely dissolved - you can use warm or room temperature water to speed this up and make sure all sugar is dissolved.
- Using the pipette or syringe, fill the empty 5th glass ¼ full with the blue sugar solution, then add ¼ of the green solution which should sit on top. Then add ¼ of the yellow solution, then ¼ of the red solution to finish. Go slowly, making sure the different layers don’t mix into each other. You should have created a rainbow!
The Science
Density is the number of particles in a given volume. When more sugar is added, more space between the water particles is filled. Adding sugar to the water increases the density of the water, so the more sugar in the solution, the greater the density.
The blue sugar solution has the most sugar so it is the densest as it has the highest number of sugar particles per 100ml of water. The densest solution stays at the bottom, with the least dense on top. The red solution has less sugar so has a lower density, and, as a result, stacks on top of the mixture with a higher density - so the differing densities separate out.
Want More Epic Experiments?
If you enjoyed this experiment and want more fun, more science, and more epic experiments, enrol now in our science enrol now in our holiday camps and science classes where kids can learn, experiment, explore, and play!
Book your Holiday workshops and Term classes today!
Schools & Nurseries
Start a Science or Little Maths club to your school/nursery
We offer tailor-made tutoring to students of Chemistry, Physics, Biology and Maths.
Latest News

We published our first book: FULL OF BRAINS!

Holiday Science Camps
AGES 4-12 YEARS
find out more and enrol

Science Term Classes
AGES 3-4 YEARS AGES 4-7 YEARS AGES 8-12 YEARS

Schools & Nursery Clubs
AGES 3-4 YEARS AGES 4-12 YEARS
Our website uses cookies to give you a better customer experience. By continuing we will assume you are happy with receiving all cookies. ACCEPT You can get more information on cookies HERE

IMAGES
VIDEO
COMMENTS
The easiest way to separate a mixture of sugar and water is to use distillation, which involves boiling the mixture until the water evaporates, leaving sugar crystals behind.
Hint – to separate the sand from water you could use a sieve. This is possible as the sand is insoluble ( doesn’t dissolve in water ). Salt and sugar are soluble ( dissolve in water ) and can be separated by evaporation. Another way to separate a mixture is by using a process called chromatography.
So, there are a few ways to separate these two ingredients, depending on your end goals: To collect just solute (sugar): heat over very low heat to evaporate as much water as possible to give yourself a saturated solution. Then crystallise sugar and filter (in the lab you'd wash with a suitable solvent and dry under vacuum)
The easiest way or process of separating a mixture of sugar and water involves a three-step procedure: distillation, evaporation, and crystallization. It’s super easy and we’ll walk you through the step-by-step process to make sure you get it right! 1) Create a mixture of sugar and water. Mix water and pure sugar in a container.
Adding a solute, such as sugar, salt, or other compounds to water will change the boiling point of the resulting solution. Try heating up your three liquids (original juice, distillate, and remaining juice) and measure their boiling points with a thermometer.
How could you separate a mixture of two things if one is soluble in water and the other is not? What is density? What machine can be used to separate things based on their density?
Gravity Separation. Magnetic Separation. Let us understand them in more detail one by one. Sedimentation is the process or technique in which insoluble heavy particles in a liquid settle down in the lower part of the container. This simple technique applies to a mixture containing one liquid and another heavier, insoluble solid component.
Mystery Mixture. Have a friend create a batch of mystery mix for you to separate. Your friend can use any or all of the following ingredients: water, sand, sugar, soil and vegetable oil. When your friend presents the mixture, split it into several samples and perform experiments to identify its individual components.
Learn how to separate components of a mixture. Calculate the percent composition of a mixture. Calculate percent recovery of sample. In this experiment, students will separate the components of a mixture containing sand (mostly SiO 2), table salt (NaCl), and calcium carbonate (CaCO 3).
Learn about density in this fun and simple science experiment. You Will Need. Sugar; Water; Food colouring; Tablespoon; 5 glasses; Pipette/syringe; The Experiment. Fill 4 glasses with water. Leave the 5th glass empty. Add 2-3 drops of food colouring to each glass of water.