Why Does Water Dissolve Salt?
Instructions, simulations.
Youtube ID: _vFB8zXTLAo

Lesson Summary Video for teachers
Note: This video is designed to help the teacher better understand the lesson and is NOT intended to be shown to students. It includes observations and conclusions that students are meant to make on their own.
Key Concepts
- The polarity of water molecules enables water to dissolve many ionically bonded substances.
- Salt (sodium chloride) is made from positive sodium ions bonded to negative chloride ions.
- Water can dissolve salt because the positive part of water molecules attracts the negative chloride ions, and the negative part of water molecules attracts the positive sodium ions.
- The amount of a substance that can dissolve in a liquid (at a particular temperature) is called the solubility of the substance.
- The substance being dissolved is called the solute, and the substance doing the dissolving is called the solvent.
Students will make a 2-D model of a salt crystal and use water molecule cutouts to show how water dissolves salt. After seeing an animation of water dissolving salt, students will compare how well water and alcohol dissolve salt. They will relate their observations to the structure of salt, water, and alcohol on the molecular level.
Students will be able to explain, on the molecular level, why water can dissolve salt. Students will be able to identify the variables in their experiment. Students will also be able to explain why a less polar liquid, such as alcohol, is not good at dissolving salt.

Be sure you and the students wear properly fitting goggles. Isopropyl alcohol is flammable. Keep it away from flames or spark sources. Read and follow all warnings on the label. Alcohol should be disposed of according to local regulations. Have students wash hands after the activity.
Materials for Each Group
- Construction paper, any color
- Tape or glue
- Isopropyl alcohol (70% or higher)
- 2 clear plastic cups
- 2 small plastic cups
- Graduated cylinder
Download All Lesson 5.3 Resources
Get the entire lesson plan and Student Activity Sheet for "Lesson 5.3 - Why Does Water Dissolve Salt?"
Download PDF DOCX | Google Doc
Online Assignments
Supplement in-class learning with interactive, multimedia-rich Google Forms lesson modules, perfect for reinforcing key chemistry concepts and scientific investigation skills.
Explore Online Assignments
Standards Alignment
5.3 Next Generation Science Standards (PDF) 5.3 Common Core State Standards (PDF)
More about Standards Alignment
Step 1 Make a model of a salt crystal.
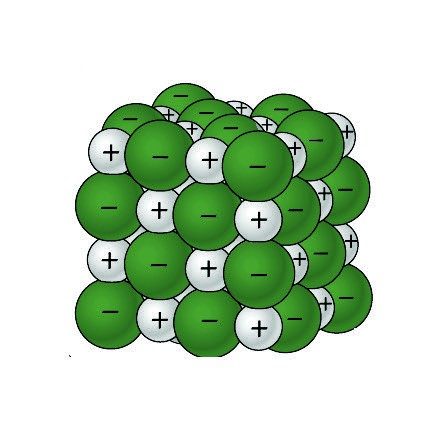
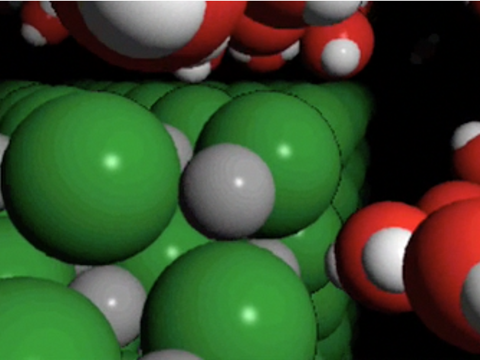
Project the image Sodium Chloride Crystal.
Remind students that the green balls represent negative chloride ions, and the gray balls represent positive sodium ions.
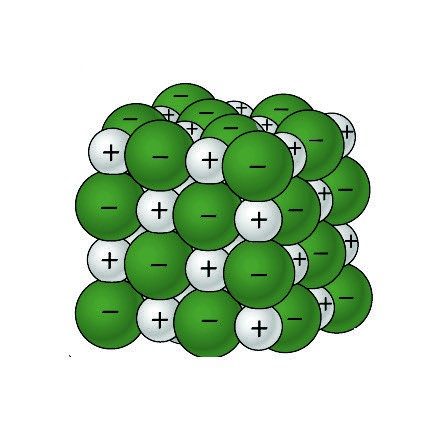
Ask students:
What is it about water molecules and the ions in salt that might make water able to dissolve salt? The positive and negative polar ends of a water molecule are attracted to the negative chloride ions and positive sodium ions in the salt.
Give each student an activity sheet.
Students will record their observations and answer questions about the activity on the activity sheet. The Explain It with Atoms & Molecules and Take It Further sections of the activity sheet will either be completed as a class, in groups, or individually, depending on your instructions. Look at the teacher version of the activity sheet to find the questions and answers.
- Lesson 5.3 Student Activity Sheet PDF | DOCX | Google Doc
All Downloads
Question to Investigate
How does salt dissolve in water?
- Activity sheet with sodium and chloride ions and water molecules
Make a model of a salt crystal
Cut out the ions and water molecules.
Arrange the ions on a piece of construction paper to represent a 2-D salt crystal. Do not tape these pieces down yet.
Step 2 Project an image and have students model what happens when salt dissolves in water.
Show students a series of four pictures to help explain the process of water dissolving salt.
Project the image Sodium Chloride Dissolving in Water.
Point out that several water molecules can arrange themselves near an ion and help remove it from the crystal. Show students that the positive area of a water molecule will be attracted to the negative chloride ion and that the negative area of a water molecule will be attracted to the positive sodium ion.
Model how water dissolves salt
Look at the pictures showing how water molecules dissolve salt. Then arrange the water molecules around the sodium and chloride ions in the correct orientation. The positive part of the water molecules should be near the negative chloride ion. The negative part of the water molecules should be near the positive sodium ion.
Move the water molecules and sodium and chloride ions to model how water dissolves salt.
Tape the molecules and ions to the paper to represent water dissolving salt.
Project the animation Sodium Chloride Dissolving in Water .
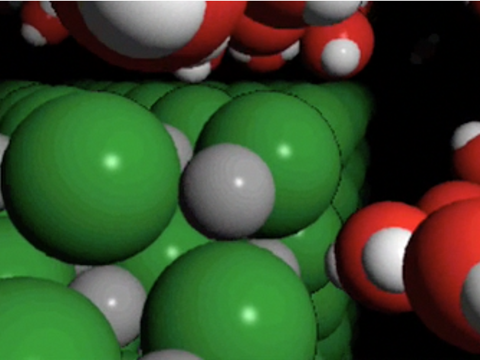
Sodium Chloride Dissolving in Water 2
Point out that the water molecules are attracted to the sodium and chloride ions of the salt crystal. Explain that the positive area of a water molecule is attracted to a negative chloride ion. The negative area water of a water molecule is attracted to a positive sodium ion. Dissolving happens when the attractions between the water molecules and the sodium and chloride ions overcome the attractions of the ions to each other. This causes the ions to separate from one another and become thoroughly mixed into the water.
Tell students that the amount of a substance that can dissolve in a liquid (at a particular temperature) is called solubility. Point out the similarity in the words dissolve and solubility. Also tell them that the substance that is dissolved is called the solute. The substance that does the dissolving is called the solvent.
The activity sheet will serve as the “Evaluate” component of each 5-E lesson plan. The activity sheets are formative assessments of student progress and understanding. A more formal summative assessment is included at the end of each chapter.
- Lesson 5.3 Activity Sheet Answers PDF | DOCX | Google Doc
Step 3 Have students conduct an experiment to find out whether water or isopropyl alcohol would be better at dissolving salt.
Ask students to make a prediction:
- Think about the polarity of water molecules and alcohol molecules. Do you think alcohol would be just as good, better, or worse than water at dissolving salt?
Discuss how to set up a test to compare how water and alcohol dissolve salt. Be sure students identify variables such as:
- Amount of water and alcohol used
- Amount of salt added to each liquid
- Temperature of each liquid
- Amount of stirring
Is alcohol just as good, better, or worse than water at dissolving salt?
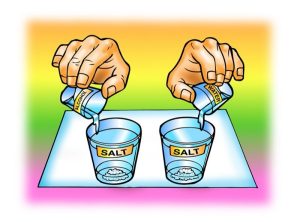
In separate cups, measure two samples of salt that weigh 5 g each.
Place 15 mL of water and alcohol into separate cups.
At the same time, add the water and alcohol to the samples of salt.
Swirl both cups the same way for about 20 seconds and check for the amount of salt dissolved.
Swirl for another 20 seconds and check. Swirl for the last 20 seconds and check.
Carefully pour off the water and alcohol from the cups and compare the amount of undissolved salt left in each cup.
Expected Results
There will be less undissolved salt in the cup with the water than the alcohol. This means that more salt dissolved in the water than in the alcohol.

Step 4 Discuss how differences in the polarity of alcohol and water explain why water dissolves salt better than alcohol.
- Is alcohol just as good, better, or worse than water at dissolving salt? Alcohol does not dissolve salt as well as water does.
- How do you know? There was more salt left behind in the cup with the alcohol.
- Think about the polarity of water and alcohol to explain why water dissolves more salt than alcohol. Have students look at the models of water and alcohol molecules on their activity sheet.
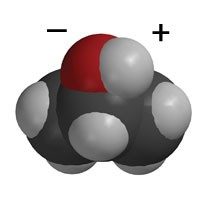
Remind students that isopropyl alcohol has an oxygen atom bonded to a hydrogen atom, so it does have some polarity but not as much as water. Since water is more polar than alcohol, it attracts the positive sodium and negative chloride ions better than alcohol. This is why water dissolves more salt than alcohol does. Another way of saying this is that the solubility of salt is greater in water than in alcohol.
Read more about polarity in Teacher Background.
- Lesson 5.3 Teacher Background PDF
All Downloads
Step 5 Have students compare the solubility of two different ionic substances in water.
Compare the solubility of the ionic substances calcium carbonate (CaCO 3 ) and sodium bicarbonate (Na 2 HCO 3 ) in water.
How could you compare the solubility of sodium bicarbonate and calcium carbonate? Students should suggest measuring equal amounts of each substance and adding equal amounts of water at the same temperature.
Do all ionic substances dissolve in water?
- Sodium bicarbonate
- Calcium carbonate
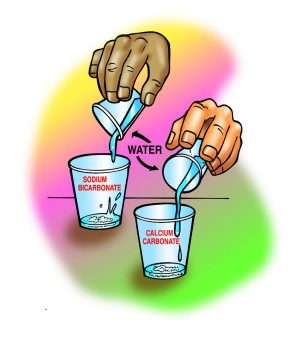
- Label two clear plastic cups Sodium Bicarbonate and Calcium Carbonate.
- Measure 2 g each of sodium bicarbonate and calcium carbonate and put them in their labeled cups.
- Measure 30 mL of water into each of two empty cups.
- At the same time, pour the water into the sodium bicarbonate and calcium carbonate cups.
- Gently swirl both cups for about 1 minute.
The sodium bicarbonate will mostly dissolve, but the calcium carbonate will not. Explain that not all ionically bonded solids dissolve in water.
Step 6 Discuss student observations
Do all ionic substances dissolve in water? How do you know? Because calcium carbonate does not dissolve in water, students should realize that not all ionic substances dissolve in water.
Explain that on the molecular level, the ions that make up calcium carbonate are attracted so strongly to each other that the attraction by water molecules cannot pull them apart.
That is a good thing because calcium carbonate is the material that seashells and bird eggs are made of. Calcium phosphate is another ionic solid that does not dissolve in water. This is also good because it is the material that bones and teeth are made of.
With enough water, sodium bicarbonate breaks apart completely into ions that are incorporated throughout the water, forming a solution. The sodium and bicarbonate ions will not settle to the bottom and cannot be filtered out of the water.
But calcium carbonate does not break up into its ions. Instead, it is just mixed in with the water. If given enough time, the calcium carbonate will settle to the bottom or can be filtered out of the water. Sodium bicarbonate dissolved in water is a good example of a solution, and undissolved calcium carbonate is a mixture, not a solution.
Note : The bicarbonate ion is different from the single atom ions such as sodium (Na + ) and chloride (Cl – ) that students have seen so far. The bicarbonate ion (HCO 3- ) is composed of more than one atom. These types of ions, called polyatomic ions, are made up of a group of covalently bonded atoms that act as a unit. They commonly gain or lose one or more electrons and act as an ion. Another common polyatomic ion is the sulfate ion (SO 2- ). This ion is part of Epsom salt as magnesium sulfate (MgSO 4 ) and many fertilizers as potassium sulfate (K 2 SO 4 ). You can decide if you would like to introduce students to these two common polyatomic ions.
What is the 5-E format?
The 5-E instructional model is an approach to teaching and learning that focuses on active engagement, inquiry-based learning, and collaboration.
Simulations for Lesson 5.3
For Students
- Lesson 5.3 Student Activity Sheet PDF | DOCX | Google Doc
For Teachers
- Lesson 5.3 Lesson Plan PDF | DOCX | Google Doc
- Lesson 5.3 Activity Sheet Answers PDF | DOCX | Google Doc
- Lesson 5.3 Teacher Background PDF
Resources for the entire Chapter 5
- Chapter 5 Student Reading PDF | DOCX | Google Doc
- Chapter 5 Test Bank PDF | DOCX | Google Doc
More from Chapter 5
Interactive Lesson Modules
- Lesson 5.3 Online Assignments Google Form
Have Questions? Visit Help Center
This lesson is part of: Chapter 5: The Water Molecule & Dissolving
Lesson 5.2: Surface Tension
Lesson 5.4: Why Does Water Dissolve Sugar?
Accept & Close The ACS takes your privacy seriously as it relates to cookies. We use cookies to remember users, better understand ways to serve them, improve our value proposition, and optimize their experience. Learn more about managing your cookies at Cookies Policy .
1155 Sixteenth Street, NW, Washington, DC 20036, USA | service@acs.org | 1-800-333-9511 (US and Canada) | 614-447-3776 (outside North America)
- Terms of Use
- Accessibility
Copyright © 2024 American Chemical Society
Science Experiments on Solubility
Many of the substances people use daily, including shampoo, gasoline and milk, are mixtures. When mixtures are homogenous, meaning the particles of each substance are mixed evenly, they create a solution. Solutions form when the attraction between the solute, a substance that dissolves, and solvent, a substance like water that does the dissolving, is greater than the particles that make up the solute. Solubility measures the amount of a solute that can dissolve in a solvent.
Saturated Solutions
Introduce solubility by testing how much a solute dissolves in water before the solution becomes too saturated. This type of experiment introduces aqueous solutions, or solutions of a substance dissolved in water, to students. The experiment can also spark a discussion about why water is able to dissolve so many substances; the attraction between water and the solute is greater than the particles of the solute. The scientific method dictates you must include a hypothesis; for example, predicting that more of one solute will dissolve than another substance. To test your hypothesis, measure 1 cup each of table salt, Epsom salt and sugar, placing each substance in a separate container. Prepare three plastic cups with 1/2 cup of distilled water each. Add 1 teaspoon of table salt to one plastic cup and stir to dissolve. Continue adding table salt to this cup in small increments until the solute will no longer dissolve. Weigh the remaining salt and subtract from the initial cup to find the amount that remains. Repeat the steps with the Epsom salt as well as sugar. Compare how much of each solute was dissolved to determine if your hypothesis was correct. You should find that some crystals of each substance remain floating in the water because the water is already saturated.
Advertisement
Article continues below this ad
More For You
Science experiments on evaporation for kids in seventh grade, science experiments with purple cabbage, a simple ph experiment to do in a class, effects of acetone on plastic, chemistry experiments with baking soda & hydrochloric acid, testing various solvents.
Water isn't the only liquid that will dissolve solids like salt and sugar. Water is considered the universal solvent because the electrical charge of its molecules attract other substances, but students might wonder if other liquids also attract and dissolve solids. Test water, rubbing alcohol, club soda, cooking oil and nail polish to determine which one is the best solvent. Create your hypothesis; for example, that nail polish will dissolve more solutes and cooking oil will be the most ineffective solvent. Prepare plastic cups with 2 teaspoons of each liquid. Measure and add 1 teaspoon of salt to each liquid and stir for 10 to 30 seconds. Record results, indicating if the salt dissolved completely, partially or not at all. Repeat the experiment with other solutes like baking soda, sugar and sand to determine if multiple substances can dissolve in particular solvents.
Results and Explanations
You will find that water is the best solvent, and heavier liquids like cooking oil are the worst. Some salt will dissolve in alcohol, but since the polarity of alcohol is not as strong as water, it is not as good a solvent. Club soda will likely dissolve more than alcohol because it contains water, but the soda is also somewhat saturated with carbon dioxide. This experiment also shows that "like dissolves like," so while salt dissolves in water because they are both polar compounds, salt will not dissolve in organic compounds like nail polish. Examine your results to see if your hypothesis was correct .
Temperature and Solubility
A common hypothesis states that hot water will dissolve more solute than cold water. Use this experiment to determine if temperature has any effect on solubility. Add a 1/2 cup of lukewarm tap water to a plastic cup. Weigh about 5 tablespoons of salt and gradually add the salt to the tap water, stirring to mix. Stop adding salt when it no longer dissolves. Repeat the mixing steps with 1/2 cup each of ice water and hot water; determine at which temperature more salt dissolves. This experiment proves that the solubility of some substances is dependent on temperature, and you will notice much more salt dissolves in hot water than in cold.
Peeps Solubility
In 1996, scholars James Zimring and Gary Falcon examined the solubility of Peeps, the bird-shaped marshmallow candy. You can duplicate a similar experiment and hypothesize that the candy is not soluble in water but will dissolve in acetone, or nail polish remover. Fill four plastic cups with 1 cup each of water, acetone, vinegar and rubbing alcohol. Submerge one Peeps candy in each liquid and observe every 20 minutes for an hour. Write down your observations. This experiment demonstrates to students the difference between what is expected versus the outcome. Many students think that candy is made from sugar, and since they know sugar will dissolve in liquids like water, they believe the candy will dissolve. The candy doesn't dissolve in any of these liquids. From these results, students can determine that the candy must be made up of other substances resistant to dissolving in liquids.
- Science Buddies: Saturated Solutions: Measuring Solubility
- Education.com: To Test the Solubility of Common Liquid Solvents
- Peep Research: Solubility Testing
Cara Batema is a musician, teacher and writer who specializes in early childhood, special needs and psychology. Since 2010, Batema has been an active writer in the fields of education, parenting, science and health. She holds a bachelor's degree in music therapy and creative writing.

IMAGES