- Skip to main content
- Skip to secondary menu
- Skip to primary sidebar
Class Notes
Free Class Notes & Study Material

Rutherford’s Model of an Atom
Last Updated on July 3, 2023 By Mrs Shilpi Nagpal
Thomson’s model of atom
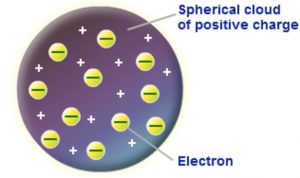
J.J. Thomson in 1904 , proposed that an atom was a sphere of positive electricity in which were embedded number of electrons. The stability of the atom was explained as a result of the balance between the repulsive forces between the electrons and their attraction towards the centre of the positive Sphere.This model is compared with a watermelon in which seeds are embedded or with a cake or pudding in which raisins are embedded. That is why this model is called as raisin pudding model a watermelon model.
This model could not explain the stability of the atom.
Rutherford Model of atom
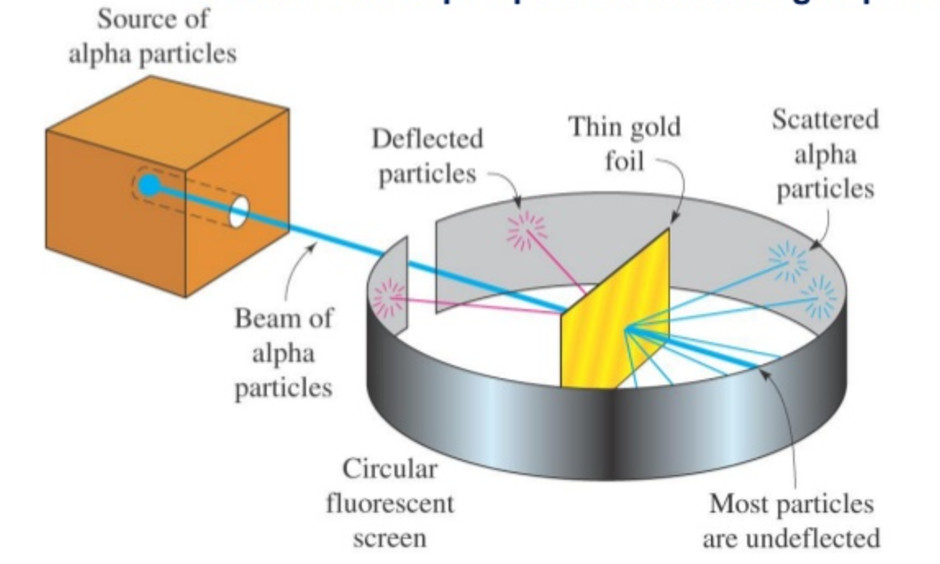
Rutherford in 1911, performed scattering experiment in which he bombarded thin walls of metals like gold, silver, Platinum or copper with a beam of fast moving Alpha particles. The source of Alpha particles was radium, a radioactive substance, placed in a block of lead. Slits were used to get a fine beam. The presence of Alpha particles at any point around the thin foil of gold after striking it was detected with the help of a circular zinc sulphide. The point at which an Alpha particle strikes this screen ,a flash of light is given out.
Observation
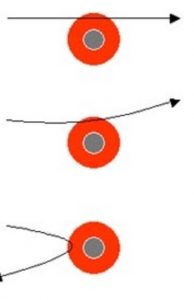
1) Most of the Alpha particles passed through the foil without undergoing any deflection.
2) Few Alpha particles underwent deflection through small angles.
3) Very few were deflected back through an angle greater than 90°
1)Since most of the Alpha particles passed through the foil without undergoing any deflection, there must be sufficient empty space within the atom.
2)Since few alpha particles were deflected through small angles and alpha particles were positively charged particles, these could be deflected only by some positive body present within the atom. The alpha particles deflected were those which passed very close to this positive body.
3)Since some alpha particles were deflected back and alpha particles are heavy particle, these could be deflected back only when they strike some heavier body inside the atom.
4)Since the number of alpha particles deflected back is very very small, this shows that the heavy body present in an atom must be occupying a very very small volume.
The small heavy positively charged body present within the atom was called nucleus.
Rutherford Model of an atom
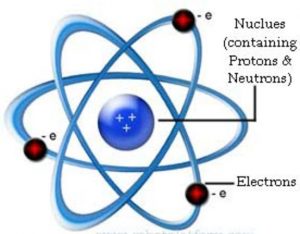
(1) Nucleus is very small in size, carries positive charge and in which the entire mass of the atom is concentrated.
(2) Since electrons have negligible mass ,the mass of the atom is mainly due to protons and neutrons.
(3) Protons and neutrons must be present in the nucleus.
(4) Extranuclear part is the space around the nucleus in which the electrons were distributed.
Drawback of Rutherford’s Model of an atom
(1) Inability to explain the stability of atom
An atom consists of a small, heavy positively charged nucleus in the centre and the electrons were revolving around it. This model was compared with the solar system in which the planets were revolving around the sun and continue to move in their fixed circular paths because the force of attraction was balanced by the centrifugal force.
According to Maxwell’s electromagnetic theory, whenever a charged particle like electron is revolving in a field of force like that of the nucleus, it loses energy continuously in the form of electromagnetic radiation. This is because when a particle is revolving, it undergoes acceleration due to change in direction even if the speed remains constant. Thus, the orbit of the revolving electron will keep on becoming smaller and smaller, following a spiral path and ultimately the electron should fall into the nucleus. The atom should collapse.
Rutherford model could not explain the stability of the atom.
(2) Inability to explain the line spectra of the elements.
(3) Inability to describe distribution of electrons and energies of electrons.
Atomic number
Atomic number of an element = Total number of protons = Total number of electrons
The atomic number is represented by Z
Atomic number is also known as Proton number because the charge on the nucleus depends upon the number of protons.
Mass number is the sum of protons and neutrons.
The mass number is represented by A.
Where A is mass number, Z is atomic number and X is the element.
Atoms of the same element having same atomic number but different mass number.
For Example:
(1) Isotopes of Hydrogen
1 H 1 1 H 2 1 H 3
(2) Isotopes of Chlorine
37 Cl 35 Cl
(3) Isotopes of Carbon
14 C 12 C 13 C
4) Isotopes of Oxygen
16 O 17 O 18 O
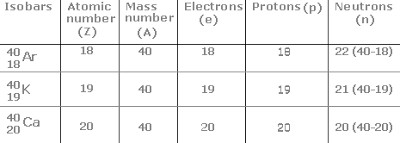
Atoms of different elements having different but same mass number are called isobars.
Atoms of different elements which contain the same number of neutrons are called isotones.

Isoelectronic
The species containing the same number of electrons are called isoelectronic.
About Mrs Shilpi Nagpal
Author of this website, Mrs. Shilpi Nagpal is MSc (Hons, Chemistry) and BSc (Hons, Chemistry) from Delhi University, B.Ed. (I. P. University) and has many years of experience in teaching. She has started this educational website with the mindset of spreading free education to everyone.
Reader Interactions
February 22, 2017 at 9:41 pm
I think that everything composed made a lot of sense.
But, what about this? suppose you added a little content? I am not saying your content is not solid, however suppose you added a headline that makes people want more? I mean Rutherford's Model Of An Atom – Chemistry, Class 11, Structure Of Atom is a little vanilla. You should look at Yahoo’s home page and note how they create post titles to get viewers interested. You might add a video or a pic or two to get readers excited about what you’ve got to say. Just my opinion, it might bring your posts a little bit more interesting.
September 8, 2020 at 8:29 am
mam you are marvellous I got a great help from your notes thank to god for this much good teachers who literally care about students irrespective of coaching mafia thanks mam.
December 20, 2020 at 11:50 am
Thanks a lot of mam
May 26, 2021 at 4:17 pm
thank you so much mam
November 1, 2022 at 1:33 pm
Thank you mam
July 14, 2023 at 8:06 pm
This helps alot in understanding the concepts easily, Thank you so much Mam
Leave a Reply
Your email address will not be published. Required fields are marked *

Describe Rutherford's scattering experiment.
Rutherford's scattering experiment: rutherford conducted a gold foil experiment where he bombarded a very thin gold foil with alpha particles. the thickness of the gold foil is ~ 100 nm with alpha particles in an evacuated chamber. around the foil, a circular fluorescent screen is coated with zinc sulphide ( zns ). when the alpha particles were passed through a slit in a lead plate, they struck the screen and produced a flash of light. rutheford explained the sub-atomic particles would deflect the alpha particles. observations of scattering experiment: most of the alpha particles passed straight without any deflection. a small fraction of the alpha particles were deflected at small angles, while few were deflected at large angles. a very few alpha particles were bounced back on the gold foil. results of scattering experiment: most of the space in an atom is empty because most of the positively charged alpha particles passed through the foil are undeflected. a few positively charged alpha particles are deflected due to a heavy positive centre in the atom. a very few alpha particles are bounced back because the nucleus is very hard and dense. the volume occupied by the nucleus is negligibly small..

State the postulates of daltons atomic theory ?
Describe JJ Thomson experiments on discovery of electrons .
List out the characters of anode rays.
Describe Rutherford model of an atom
Describe the properties of cathode rays
State the conclusion drawn by Alpha ray scattering experiment of Rutherford
___ thin foil was used by Rutherford for a l p h a - scattering experiment.
In Rutherford alpha-scattering experiment, a foil of ____________ was used.

- Chemistry Class 9 Notes
- Physical Chemistry
- Organic Chemistry
- Inorganic Chemistry
- Analytical Chemistry
- Biochemistry
- Chemical Elements
- Chemical Compounds
- Chemical Formula
- Real life Application of Chemistry
- Chemistry Class 8 Notes
- Chemistry Class 10 Notes
- Chemistry Class 11 Notes
- Chemistry Class 12 Notes
Rutherford’s Alpha Scattering Experiment
Rutherford’s Alpha Scattering Experiment is the fundamental experiment done by Earnest Rutherford’s Alpha Scattering Experiment that gives the fundamental about the structure of the atom. Rutherford in his experiment directed high-energy streams of α-particles from a radioactive source at a thin sheet (100 nm thickness) of gold. Then the deflection of these alpha particles tells us about the structure of atoms.
In this article, we will study about constituents of atoms, Rutherford’s Alpha Scattering Experiment,
What are Constituents of an Atom?
An atom consists of Electrons, Protons, and Neutrons are the fundamental particles or sub-atomic particles that build the structure of an atom. Let us understand each term.
- Electron: In 1897, J. J. Thomson discovered negatively charged particles towards the anode, these rays are emitted by the cathode in a cathode ray experiment. Then these negatively charged particles are proposed as Electrons .
- Protons: In 1886, Ernest Goldstein discovered that an anode emitted positively charged particles with a different condition in the same tube, known as Canal rays or as Protons .
- Neutrons: A subatomic particle with no charge and a mass equivalent to protons in the nucleus of all atoms was discovered by J. Chadwick. These neutrally charged particles are termed Neutrons .
The image added below shows the structure of an atom.
Learn more about, Atomic Structure
.jpg)
Structure of Atom
Isotopes are the elements that have the same atomic number but different mass. e.g. Isotopes of the Hydrogen atoms are Protium ( 1 H 1 ), Deuterium ( 2 H 1 ) and Tritium( 3 H 1 ). Isotopes of the Carbon atoms are 12 C 6 , 13 C 6 , 14 C 6 .
Isobars are the elements that have different atomic number but have same mass number. e.g. 19 K 40 , 18 Ar 40 , 20 Ca 40 , here all the elements having same mass number hence they are isobars.
He conduct an experiment by bombarding alpha particles into a thin sheet of gold and then notices their interaction with the gold foil and trajectory or path followed by these particles.
.webp)
In the experiment, Rutherford passes very high streams of alpha-particles from a radioactive source i.e. alpha-particle emitter, at a thin sheet of100 nm thickness of gold. In order to examine the deflection produced by the alpha particles, he placed a screen of fluorescent zinc sulphide around the thin gold foil. Rutherford made certain observations that oppose Thomson’s atomic model.
Observations of Rutherford’s Alpha Scattering Experiment
The observations of Rutherford’s Alpha Scattering Experiment are:
- First, he observe that most of the α-particles that are bombarded towards the gold sheet pass away the foil without any deflection, and hence it shows most of the space is empty.
- Out of all, some of the α-particles were deflected through the gold sheet by very small angles, and hence it shows the positive charge in an atom is non-uniformly distributed. The positive charge is concentrated in a very small volume in an atom.
- Very few of the alpha-particles(1-2%) were deflected back, i.e. only a very less amount of α-particles had nearly 180° angle of deflection. this shows that the volume occupied by the positively charged particles is very small as compared to the total volume of an atom.
Rutherford Atomic Model
Rutherford proposed the atomic structure of elements, on the basis of his experiment. According to Rutherford’s atomic model:
- Positively charged particle was concentrated in an extremely small volume and most of the mass of an atom was also in that volume. He called this a nucleus of an atom.
- Rutherford proposed that there is negatively charged electrons around the nucleus of an atom. the electron surrounding the nucleus revolves around it in a circular path with very high speed. He named orbits to these circular paths.
- Nucleus being a densely concentrated mass of positively charged particles and electrons being negatively charged are held together by a strong force of attraction called electrostatic forces of attraction.
Learn about, Rutherford Atomic Model
Limitations of Rutherford Atomic Model
The Rutherford atomic model is failed to explain certain things.
- According to Maxwell, an electron revolving around the nucleus should emit electromagnetic radiation due to accelerated charged particles emit electromagnetic radiation. but Rutherford model says that the electrons revolve around the nucleus in fixed paths called orbits. The radiation would carry energy from the motion which led to the shrinking of orbit. Ultimately electrons would collapse inside the nucleus.
- As per the Rutherford model, calculations have shown that an electron would collapse in the nucleus in less than 10 -8 seconds. So Rutherford model has created a high contradiction with Maxwell’s theory and Rutherford later could not explain the stability of an atom.
- Rutherford also did not describe the arrangement of electrons in the orbit as one of the other drawbacks of his model.
Regardless of seeing the early atomic models were inaccurate and failed to explain certain experimental results, they were the base for future developments in the world of quantum mechanics.
Sample Questions on Rutherford’s Alpha Scattering Experiment
Some sample questions on Rutherford’s Alpha Scattering Experiment is,
Q1: Represent Element ‘X’ which contains 15 electrons and 16 neutrons.
Atomic number of element = No. of electron = 15 Mass number of element = no. of electrons + no. of neutrons = 15 + 16 = 31 Correct representation of element X is 31 X 15 .
Q2: Name particle and give its location in the atom which has no charge and has a mass nearly equal to that of a proton.
The particle which has no charge and has a mass nearly equal to that of a proton is a neutron and it is present in the nucleus of the atom.
Q3: An atom has both electron attribute negative charge and protons attribute positive charge but why there is no charge?
Positive and negative charges of protons and electrons are equal in magnitude, they cancel the effect of each other. So, the atom as a whole is electrically neutral.
Q4: What is Valency of Sodium Atom (Na)?
The atomic number of sodium = 11. Electronic configuration (2, 8, 1). By losing one electron it gains stability hence its valency is 1.
Q5: Which property do the following pairs show? 209 X 84 and 210 X 84
Atomic number of X is the same hence the pair shows an isotopic property. So, 209 X 84 and 210 X 84 are isotopes.
Dalton’s Atomic Theory Thomson’s Atomic Model Quantum Numbers
Rutherford’s Alpha Scattering Experiment FAQs
What is name of atom which has one electron, one proton and no neutron.
Atom with one electron, one proton and no neutron is Hydrogen, ( 1 H 1 ).
What is Ground State of an Atom?
It is the state of an atom where all the electrons in the atom are in their lowest energy state or levels is called the ground state.
What was Rutherford’s Alpha Particle Scattering Experiment?
Rutherford’s Alpha Particle Scattering Experiment is the fundamental experiment that gives the basic structure of an atom.
What was Conclusion of Rutherford’s Alpha Scattering Experiment?
Conclusion of Rutherford’s Alpha Scattering Experiment is, Atom is largely empty and has a heavy positive-charged body at the center called the nucleus. The central nucleus is positively charged and the negatively-charged electrons revolve around the nucleus.
Similar Reads
- School Chemistry
- School Learning
- Chemistry-Class-9
- Physical-Chemistry
Improve your Coding Skills with Practice
What kind of Experience do you want to share?

Rutherford Scattering Experiment
- Updated by Scienly
- On September 10, 2024
In this chapter, we will understand Rutherford’s scattering experiment and its observations and conclusions. In order to understand the arrangement of charged particles like electrons and protons in an atom, the British scientist, Ernest Rutherford and his co-workers in 1911 carried out a series of experiments using alpha particles known as Rutherford’s alpha scattering experiments. This experiment is based on the alpha particles (helium nuclei) experiment.
The Rutherford’s alpha particle scattering experiment is one of the most important experiments in the history of atomic physics. It laid the foundation for the modern understanding of the atom, challenging earlier models and introducing the concept of an atomic nucleus.
Before Rutherford’s experiment, J. J. Thomson had given the first model of an atom in 1904, also known as “plum pudding model of atom”. In this model, he proposed that an atom may be considered as a sphere of positively charge particles in which the negatively charged particles called electrons are embedded to make the atom as a whole neutral.
This model was discarded in 1911 on both theoretical and experimental due to the Rutherford’s atomic model. Now let’s understand Rutherford scattering experiment step by step.
Rutherford’s Alpha Particle Scattering Experiment
In this experiment, Ernest Rutherford bombarded a beam of alpha particles with a very thin gold foil. The thickness of gold foil is approximately 0.00006 cm. The alpha particles were emitted from the radioactive substances such as radium or polonium. An alpha particle is a doubly ionized helium atoms or ions with two units of positive charge, mass number is equal to 4 and missing two electrons. It is represented as +2 He 4 or +2 α 4 .
The pictorial representation of Rutherford’s scattering experiment is shown in the below figure.

From the above figure, it is clear that he produced a narrow beam of alpha particles from a radioactive substance like radium placed in a lead block and then passed it through a thin sheet of gold foil. A movable circular screen coated with Zinc Sulphide (ZnS) screen is placed around the gold foil in order to detect alpha particles after scattering.
When these alpha particles hit the screen, it produced a tiny flash of light (scintillation) on the screen, which could be observed through movable a microscope.
Observations from Alpha Particle Scattering Experiment
The following observations were made from the alpha particle scattering experiment:
- Most of the alpha particles (nearly 99%) passed through the gold foil with little to no deflection. In other words, most of the alpha particle moved in the straight path with no deflection. This means that most of the space inside the atom is empty, as shown in the above figure.
- Some of the alpha particles deflected with small angle, meaning that the positive charge of an atom occupies a tiny space.
- A very few alpha particles (approx. 1 in 20,000) deflected back through the deflection of 180 degrees. This means that the whole positive charge and mass of the atom is concentrated in a very small volume inside the atom.
Thomson’s atomic model could not explain these observations. According to Thomson’s model of atom, positive charge and mass inside an atom are uniformly distributed throughout its volume.

Conclusion from Rutherford Scattering Experiment
After performing a series of scattering experiments, Rutherford concluded that:
- Most of the space inside an atom is empty because most of the alpha particles passed through the gold foil undeflected.
- There must be the presence of a heavy positively charged mass at the center of an atom because some of the alpha particles are deflected by a certain angle.
- A heavy positively charged mass at the center of the atom is very small, which he named as nucleus.

On the basis of these observations, Sir Ernest Rutherford proposed the nuclear model of atom, which you will study in the next chapter.
Related Posts

Electromagnetic Spectrum: Definition, Examples
- October 28, 2024

Wave Nature of Electromagnetic Radiation
- October 23, 2024
What are Isotones: Definition, Examples
- October 19, 2024

What are Isobars: Definition, Examples, Difference
- October 5, 2024
- Alpha-Particle Scattering and Rutherford’s Nuclear Model of Atom
In 1911, Rutherford, along with his assistants, H. Geiger and E. Marsden, performed the Alpha Particle scattering experiment , which led to the birth of the ‘nuclear model of an atom ’ – a major step towards how we see the atom today.
Suggested Videos
J.j thomson’s plum-pudding model.
In 1897-98, the first model of an atom was proposed by J.J. Thomson. Famously known as the Plum-pudding model or the watermelon model, he proposed that an atom is made up of a positively charged ball with electrons embedded in it. Further, the negative and positive charges were equal in number , making the atom electrically neutral.
Figure 1 shows what Thomson’s plum-pudding model of an atom looked like. Ernest Rutherford, a former research student working with J.J. Thomson, proposed an experiment of scattering of alpha particles by atoms to understand the structure of an atom.
Rutherford, along with his assistants – H. Geiger and E. Marsden – started performing experiments to study the structure of an atom. In 1911, they performed the Alpha particle scattering experiment, which led to the birth of the ‘nuclear model of an atom’ – a major step towards how we see the atom today.

Figure 1. Source: Wikipedia
Browse more Topics under Atoms
- Atomic Spectra
- Bohr Model of the Hydrogen Atom
The Alpha Particle Scattering Experiment
They took a thin gold foil having a thickness of 2.1×10 -7 m and placed it in the centre of a rotatable detector made of zinc sulfide and a microscope. Then, they directed a beam of 5.5MeV alpha particles emitted from a radioactive source at the foil. Lead bricks collimated these alpha particles as they passed through them.
After hitting the foil, the scattering of these alpha particles could be studied by the brief flashes on the screen. Rutherford and his team expected to learn more about the structure of the atom from the results of this experiment.
Source: Wikipedia
Observations
Here is what they found:
- Most of the alpha particles passed through the foil without suffering any collisions
- Around 0.14% of the incident alpha particles scattered by more than 1 o
- Around 1 in 8000 alpha particles deflected by more than 90 o
These observations led to many arguments and conclusions which laid down the structure of the nuclear model on an atom.
Conclusions and arguments
The results of this experiment were not in sync with the plum-pudding model of the atom as suggested by Thomson. Rutherford concluded that since alpha particles are positively charged, for them to be deflected back, they needed a large repelling force. He further argued that for this to happen, the positive charge of the atom needs to be concentrated in the centre, unlike scattered in the earlier accepted model.
Hence, when the incident alpha particle came very close to the positive mass in the centre of the atom, it would repel leading to a deflection. On the other hand, if it passes through at a fair distance from this mass, then there would be no deflection and it would simply pass through.
He then suggested the ‘nuclear model of an atom’ wherein the entire positive charge and most of the mass of the atom is concentrated in the nucleus. Also, the electrons are moving in orbits around the nucleus akin to the planets and the sun. Further, Rutherford also concluded from his experiments that the size of the nucleus is between 10 -15 and 10 -14 m.
According to Kinetic theory, the size of an atom is around 10 -10 m or around 10,000 to 100,000 times the size of the nucleus proposed by Rutherford. Hence, the distance of the electrons from the nucleus should be around 10,000 to 100,000 times the size of the nucleus.
This eventually implies that most of the atom is empty space and explains why most alpha particles went right through the foil. And, these particles are deflected or scattered through a large angle on coming close to the nucleus. Also, the electrons having negligible mass, do not affect the trajectory of these incident alpha particles.
Alpha Particle Trajectory
The trajectory traced by an alpha particle depends on the impact parameter of the collision. The impact parameter is simply the perpendicular distance of each alpha particle from the centre of the nucleus. Since in a beam all alpha particles have the same kinetic energy, the scattering of these particles depends solely on the impact parameter.
Hence, the particles with a small impact parameter or the particles closer to the nucleus, experience large angle of scattering. On the other hand, those with a large impact parameter suffer no deflection or scattering at all. Finally, those particles having ~zero impact parameter or a head-on collision with the nucleus rebound back.
Coming to the experiment, Rutherford and his team observed that a really small fraction of the incident alpha particles was rebounding back. Hence, only a small number of particles were colliding head-on with the nucleus. This, subsequently, led them to believe that the mass of the atom is concentrated in a very small volume.
Electron Orbits
In a nutshell, Rutherford’s nuclear model of the atom describes it as:
- A small and positively charged nucleus at the centre
- Surrounded by revolving electrons in their dynamically stable orbits
The centripetal force that keeps the electrons in their orbits is an outcome of:
- The positively charged nucleus and
- The negatively charged revolving electrons.
Solved Example for You
Question: Rutherford, Geiger and Marsden, directed a beam of alpha particles on a foil of which metal
Solution: Gold
Customize your course in 30 seconds
Which class are you in.

- Shell Model
- Frank Hertz Experiment
- Effects of Radiation
- Ionizing Radiation
- Quantum Mechanics
One response to “Atomic Spectra”
i really have learnt alot, but its is difficult for me to register because my country(Nigeria) is not on the listed countries. pls kindly include if you can
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Download the App

Talk to our experts
1800-120-456-456
Explain Rutherford’s \[\alpha \] ray scattering experiment with a neat diagram.
- Question Answer
- Explain Rutherfords alpha ray ...

Repeaters Course for NEET 2022 - 23

IMAGES
VIDEO
COMMENTS
According to the Rutherford atomic model: The positive charge and most of the mass of an atom is concentrated in an extremely small volume. He called this region of the atom as a nucleus. Rutherford’s model proposed that the negatively charged electrons surround the nucleus of an atom. He also claimed that the electrons surrounding the ...
Rutherford Model of an atom. (1) Nucleus is very small in size, carries positive charge and in which the entire mass of the atom is concentrated. (2) Since electrons have negligible mass ,the mass of the atom is mainly due to protons and neutrons. (3) Protons and neutrons must be present in the nucleus. (4) Extranuclear part is the space around ...
List out the characters of anode rays. Describe Rutherford model of an atom. Describe the properties of cathode rays. State the conclusion drawn by Alpha ray scattering experiment of Rutherford. Q. ___ thin foil was used by Rutherford for alpha - scattering experiment. Q. In Rutherford alpha-scattering experiment, a foil of ____________ was used.
Rutherford Atomic Model. Rutherford proposed the atomic structure of elements, on the basis of his experiment. According to Rutherford’s atomic model: Positively charged particle was concentrated in an extremely small volume and most of the mass of an atom was also in that volume. He called this a nucleus of an atom.
The Rutherford’s alpha particle scattering experiment is one of the most important experiments in the history of atomic physics. It laid the foundation for the modern understanding of the atom, challenging earlier models and introducing the concept of an atomic nucleus. Before Rutherford’s experiment, J. J. Thomson had given the first model ...
Rutherford, along with his assistants – H. Geiger and E. Marsden – started performing experiments to study the structure of an atom. In 1911, they performed the Alpha particle scattering experiment, which led to the birth of the ‘nuclear model of an atom’ – a major step towards how we see the atom today. Figure 1. Source: Wikipedia.
Maths. ₹ 38,500 (9% Off) ₹ 35,000 per year. EMI starts from ₹2,916.67 per month. Select and buy. Explain Rutherford’s \\ [\\alpha \\] ray scattering experiment with a neat diagram.. Ans: Hint: Rutherford’s experiment was regarding the proposal of the structure of atoms. He used a metal sheet in his experiment which was bombarded with ...
In his alpha particle scattering (gold foil) experiment, Rutherford used alpha particles along with a zinc sulphide covered screen. The alpha particles were fired as a beam of 5.5MeV against a very thin gold foil sheet with 2.1 × 10-7 m thickness. It was assumed that all of the alpha particles would travel through the gold foil without being ...
Alpha Scattering. In 1909 a group of scientists were investigating the Plum Pudding model. Physicist, Ernest Rutherford was instructing two of his students, Hans Geiger and Ernest Marsden to carry out the experiment. They were directing a beam of alpha particles (He 2+ ions) at a thin gold foil. They expected the alpha particles to travel ...
and Marsden with the scattering of alpha particles by thin gold and silver foils (Phil. Mag. 25. 605 (1913), Figure 1). Performance of similar experiments in an undergraduate laboratory is not only of historical interest, but serves to demonstrate how scattering experiments provide the physicist with a powerful investigative technique.