An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Role of Surgery in Metastatic Breast Cancer: Insights from a Narrative Review
Maha ahmed alamodi alghamdi, syed esam mahmood.
- Author information
- Article notes
- Copyright and License information
Correspondence: Syed Esam Mahmood, Abha, 62529, Saudi Arabia, Tel +966550484344, Email [email protected]
Received 2023 Jan 24; Accepted 2023 Apr 27; Collection date 2023.
This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution – Non Commercial (unported, v3.0) License ( http://creativecommons.org/licenses/by-nc/3.0/ ). By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms ( https://www.dovepress.com/terms.php ).
Metastatic breast cancer is difficult to cure and has a worse prognosis with higher rates of mortality. Recently, breast surgery is believed to improve the survival rates among these women, but due to limited evidence, definite conclusions cannot be made. Therefore, we undertook this narrative review to synthesize the evidence from existing studies to assess the effectiveness of locoregional surgery and surgery of metastatic sites in improving the outcomes among women diagnosed with metastatic cancer disease along with the summary of current treatment guidelines. We reviewed PubMed and Embase and included both observational studies and randomized controlled trials (RCTs) that were published in English between 2000 and 2021. Outcomes were either survival, quality of life, toxicity related to local treatment assessed by mortality at the end of one month, progression-free survival, and breast cancer-specific survival. The main effect size assessed was hazard ratio with their 95% CIs. After literature search, we found 8 observational studies and 3 RCTs. The findings of the observational studies revealed that breast cancer surgery improves survival from 30% to 50% among women. However, findings from RCTs were mixed for local and distant progression survival. Surgery improved the local progression-free survival but worsened the distant progression-free survival. Besides, there was no effect of breast surgery on quality of life. Regarding the surgery of metastatic site, studies are complex with mixed findings and variation in survival depending upon the type of metastatic site and response to initial systematic therapy and other factors. Based on the existing mixed evidence, it is not possible to make firm and definite conclusions about the effectiveness of breast surgery in improving the survival or quality of life among women with metastatic breast cancer. In future, more RCTs are required with a larger sample size to confirm the findings of observational studies.
Keywords: breast surgery, metastatic breast cancer, women, narrative review
Introduction
Breast cancer is a highly prevalent cancer in women across the world as it contributes to 25% of all diagnosed cancers. 1 It is the second most common cause of cancer-related mortality in the United States. 2 It is estimated that one out of every eight women will be diagnosed with breast cancer in their lifetime and the most commonly affected age group is 55 to 64 years. 3 Each year, approximately 1.67 million cases of breast cancer are identified globally. 4 Of the total breast cancer cases, 20% to 30% end up having metastatic breast cancer, which eventually causes 400, 000 to 50,000 deaths annually across the world. 5 At the time of initial diagnosis, around 3.5% of women with breast cancer had already developed distant metastasis in the United States and this proportion is even higher in resource-poor settings. 6 This implies that annually around 50,000 women will be diagnosed with metastatic breast cancer. 7
While it may be challenging to have a complete cure for metastatic breast cancer, women can now live relatively longer than before due to various treatment modalities. This has increased the survival rate of five years from 10% in 1970 to approximately 40% after 1995. 8 Also, the existing evidence reveals that median survival has improved from 20 to 26 months over the last two decades. 9 However, this increased life expectancy may enhance the risk of symptoms among those women who have not undergone surgical removal of the breast. Although it is necessary to treat breast cancer, sometimes treatment can negatively affect the women’s quality of life. 10 This is perhaps due to fear of developing the disease again, decrease sexuality, and loss of job.
Since metastatic breast cancer is considered an incurable disease with a worse prognosis, usually patients are provided with palliation and systematic therapy. 11 Typically, breast surgery is performed if a woman presents with symptoms. Over the last many decades, it was believed that after metastasis, aggressive local treatment is not beneficial and therefore should not be considered as the treatment of choice. 12 However, evidence from retrospective observational studies and few intervention studies suggest that patients with metastatic breast cancer may benefit from surgery and removal of the breast tumor. 12 This evidence-primarily comes from observational studies, which are subject to confounding and selection bias issues. 13 Therefore, it is important to review the evidence both from observational and interventional studies before making any conclusions about the role of breast surgery in metastatic breast cancer. Further, the evidence generally considers systematic therapy as the main treatment modality in case of metastasis. However, locoregional surgery by removing the breast and axillary tissues along with treating or doing surgery of meta-static site may reduce the symptoms and prevent cancer-related adverse outcomes. To provide up-to-date evidence, we performed a narrative review of both observational and interventional studies carried out to synthesize the findings regarding the efficacy of breast surgery in treating metastatic breast cancer. In addition, we also reviewed the evidence regarding the role of surgery of metastatic sites in preventing cancer-related complications followed by providing a summary of current guidelines in managing metastatic breast cancer.
This narrative review addresses the research question of what is the benefit of breast surgery (mastectomy: removal of the complete breast along with lymph nodes, nipple and areola or lumpectomy: removing breast tissue and tumor but preserving nipple and areola) among women diagnosed with metastatic breast cancer. Any study either observational or intervention study that assessed the effectiveness of breast surgery in improving the survival or quality of life among women with metastatic breast cancer, published in the English language between 2000 and 2021 was included. We searched the databases such as PubMed and Embase by using appropriate search terms such as “Breast surgery” OR” mastectomy” OR “lumpectomy” AND “Metastatic disease” AND “Survival” AND “observational studies” OR” intervention studies” AND “Quality of Life”. The randomized controlled trials that compared breast surgery with systematic therapy including endocrine therapy, chemotherapy, radiotherapy, biologic therapy, and supportive care against only systematic therapy were included. Outcomes were either survival, quality of life, toxicity related to local treatment assessed by mortality at the end of one month, progression-free survival, and breast cancer-specific survival ( Table 1 ).
Methodology Applied for the Development of This Narrative Review
Study Results
Indication of locoregional surgery (breast and axilla) in metastatic breast cancer: evidence from observational studies.
Overall, the existing evidence from retrospective observational studies reveals a beneficial effect of breast surgery in improving the survival of women ( Table 2 ). For instance, a study by Khan et al in 2002 on 16,023 patients found that breast surgery improved the survival of women by almost 40% (HR: 0.61, 95% CI: 0.58 to 0.65) with statistically significant results. 6 In 2006, Babiera et al conducted a retrospective analysis of 224 patients and 37% of those underwent surgery. 14 The findings of the study demonstrated that breast surgery improved survival by 50% (HR: 0.51, 95% CI: 0.21 to 1.19); however, the results were not statistically significant. 14 These findings were in agreement with a study by Rapiti et al who performed the study in the same year (2006) on 300 patients. 15 Around 42% of the women underwent surgery and authors found surgical treatment reduces the mortality by 40% (HR: 0.60, 95% CI: 0.4 to 1.0) with statistically non-significant results as shown in Table 2 . 15
Summary of Findings Regarding the Effectiveness of Locoregional Breast Surgery in Women Diagnosed with Metastatic Breast Cancer
Likewise, in 2007, Fields et al undertook a study on 409 patients and 46% of women underwent surgery. 16 Authors found that surgery improved survival by almost 50% with statistically significant results (HR: 0.53, 95% CI: 0.42 to 0.67). These findings were analogous to the study by Babiera et al, which provided evidence for the protective effect of breast surgery against mortality. 14 This series of observational studies were followed by subsequent studies from 2006 to 2010 with almost consistent findings for the effect size of the hazard ratio. For instance, in 2007, Gnerlich et al undertook a huge study on 9734 women and 47% of them underwent surgery. 17 Findings revealed that breast surgery reduces mortality by almost 40% with significant and reasonably precise results as indicated by narrow confidence intervals (HR: 0.63, 95% CI: 0.60 to 0.66). 17 In the next year (2008), Blanchard found similar results, however, with wide 95% CIs most likely due to the small sample size of 395 (HR: 0.71, 95% CI: 0.56 to 0.91). 18 These findings were confirmed by Ruiterkam et al and Neuman et al, where surgery was found to reduce mortality by almost 30% to 40%. However, as opposed to Ruiterkam et al (HR: 0.62, 95% CI: 0.51 to 0.76), Neuman et al did not find statistically significant results (HR: 0.71, 95% CI: 0.47 to 1.06). 19 , 20 These results are analogous to subsequent retrospective studies conducted during the same decade, which provide similar conclusions about the role of breast surgery in improving the survival of women diagnosed with metastatic cancer. 21 , 22
Indication of Locoregional Surgery (Breast and Axilla) in Metastatic Breast Cancer: Findings from Interventional Studies
While the observational studies suggest the protective role of surgery in metastatic cancer, these studies are not free of limitations. Due to limitations of internal threats to validity resulting from selection and information bias, and unmeasured confounding, it is crucial to review the evidence from interventional studies before making any firm conclusions. This is because randomized controlled trials (RCTs) control for known and unknown confounders and such designs are considered the ideal and gold standard to address the issues of unmeasured confounding. Recently, only four RCTs are conducted to compare the locoregional surgery plus systematic treatment against only systematic treatment in improving the survival, quality of life, local progression-free survival, distant progression-free survival, breast cancer-specific mortality, and toxicity due to local treatment among women diagnosed with metastatic breast cancer. 23 , 24 If we apply the PICOS framework to such RCTs, the population would be women diagnosed with breast cancer, intervention would be breast surgery plus systematic treatment, the comparison would be systematic treatment, the outcome is the survival or mortality, and setting would be inpatient or outpatient departments. For example, Badwe et al and Soran et al undertook two RCTs on 624 women in 2015 and 2016, respectively, to compare the breast surgery plus systematic treatment with only systematic treatment ( Table 2 ).
Badwe et al conducted a study in India on women who responded to systematic therapy initially, 24 whereas Soran et al carried out a study on women without any previous systematic treatment. 23 Authors found that overall survival at two years improved by almost 20% in the intervention group; however, results were not statistically significant (HR: 0.83, 95% CI: 0.53 to 1.31). 23 , 24 The risk of death was 511 per 1000 in the comparison group, whereas it was 448 per 1000 in the intervention group with a range of 318 to 608 per 1000. 23 , 24 However, the authors did not report the quality of life as an outcome of interest. 23 , 24 Regarding local progression-free survival at two years, findings were statistically significant (HR: 0.22, 95% CI: 0.08 to 0.57), which reveals that breast surgery improved the local progression-free survival by almost 80%. 23 , 24 However, the results were inverse for the distant progression-free survival (HR: 1.42, 95% CI: 1.08 to 1.86), which indicate that surgery may worsen the distant progression-free survival. 24 On the other hand, Radisic et al published an RCT in 2020 to compare the effect of primary surgery on patient-reported quality of life versus no surgery among women with metastatic breast cancer. 25 Authors randomized 90 women to either surgery or a systematic therapy without surgery. 25 The study demonstrated no statistically significant difference in the quality of life as reported by the patients between the intervention and control group. 25 The authors concluded that primary surgery neither improves nor deteriorates the quality of life ( Table 2 ). In 2020, a multi-center trial was conducted on 256 patients diagnosed with stage IV breast cancer who already had undergone systematic therapy for 4 to 8 weeks and were randomized to either systemic therapy or local surgery with or without radiation followed by resuming the systematic therapy. 26 The authors did not find any difference in the three-year survival between the two groups (HR: 1.09, 95% CI: 0.80 to 1.49). Although women receiving only systematic therapy were at a higher risk of locoregional progression, they experienced almost equal or even better quality of life-related outcomes when compared with women receiving local surgery. 26 Another trial by Eastern Cooperative Oncology Group ‐ USA is not completed yet and probably will end by 2025. This trial compares early surgery against standard palliative therapy to treat women with advanced breast cancer. Based on the findings from limited RCTs with inconsistent findings, it is unclear whether breast surgery is effective for metastatic breast cancer until evidence is drawn from more well-designed and well-executed RCTs in the future on the larger sample size.
Sites of Metastasis in Breast Cancer and Evidence for the Indication of Surgery of Metastatic Site
There could be various sites where breast cancer can spread such as the brain, eyes, bones, lungs, liver, ovaries, and gastrointestinal tract. Metastasis to these sites is sometimes considered an emergency, leading to making decisions about surgically removing the metastatic site. However, the evidence to perform surgery or provide any other treatment differ by the site of metastasis. For example, for patients with metastasis to the brain, surgical resection, stereotactic radiosurgery, or radiotherapy of the whole brain should be considered regardless of the patient is having symptoms or not. 27 However, this should be done either before or concurrently with systematic treatment. However, there are some exceptions to this for selected patients with restricted intracranial disease due to HER2-positive breast cancer, where only systematic therapy may be considered an option in some cases. Meanwhile, radiation therapy is used for patients with multiple metastatic deposits or extensive leptomeningeal disease. However, for patients with the limited intracranial disease, stereotactic radiosurgery is used whenever possible rather than the radiation of the whole brain to reduce the risk of toxicity. 28
On the other hand, metastasis involving ophthalmic structures ranges from 5% to 38%, with the commonly involved site in metastatic breast cancer being choroid followed by iris, optic nerve, orbital bone, retina, extraocular muscles, vitreous body, ciliary body, an optical disc. 29 , 30 There should be multidisciplinary decisions for local therapy when metastasis involves choroid. 31 In case of metastasis involving ophthalmic structures, a brain CT scan and MRI are also recommended to figure out any concurrent brain metastasis. As far as treatment options for choroidal metastasis are concerned, there are various options. For example, among asymptomatic patients, systematic therapy with careful follow-up and observation is recommended. Whereas, in symptomatic patients, palliative external-beam radiation therapy after sparing the lens and the anterior chamber is considered a safe and effective therapy. For instance, evidence from a prospective study conducted in Germany revealed that visual function improved in 36% of the patients and stabilized in 50% among those who were treated with external-beam radiation therapy (40 Gy) after being diagnosed with choroidal metastasis. 32 On the follow-up of these patients, complete and partial regression was documented in 38% and 44% of the patients, respectively. Likewise, short courses of external-beam radiation therapy may also be useful and improve the visual symptoms and visual acuity as found in one observational study on 123 patients. Other local treatment alternatives could be plaque brachytherapy, proton therapy, or intravitreal injection of anti-vascular endothelial growth factors. 33 However, the data on these therapies are limited and based on case reports or case series. 33
In case of metastasis to bone, indications for local treatment include any bone disease or pathologic fracture, compression of the spinal cord, decreased movement or pain, and disability. Bony metastasis usually responds well to systematic therapy and for asymptomatic patients without evidence of impending fracture, there is no clear evidence and role for resection. However, in the case of painful metastasis, a large body of literature supports the use of the short course, local-fixed external-beam radiation therapy in single or multiple fractions. 34 , 35 Similarly, metastasis to the lungs typically does not need to be intervened locally until patients develop some life-threatening symptoms due to internal or external obstruction to superior vena cava where local treatments may benefit. 36–38 In some cases, pulmonary resection can be done for diagnostic as well as palliative purposes because solitary pulmonary nodules may not be actually breast cancer metastasis. There is no valid and robust data suggestive of resecting the pulmonary tissues in case of metastatic breast cancer except date from case series suggest that it may improve 5-year survival from 30% to 80%, and median survival duration may increase from 40 to 100 months among selective breast cancer patients. 39–42 Findings from a meta-analysis on 2000 patients documented a 5-year survival rate of 46% after resecting isolated metastasis of lung tissue. 43
In case of metastasis to the liver, where more than 50% of the breast cancer can metastasize and most likely present with the disseminated disease with poor prognosis than metastasis to bones or soft tissues with 5% to 10% have isolated involvement of liver. 44 , 45 Usually, local management of liver metastasis is done in case symptoms such as pain, bleeding not treated by medical therapy, or in case of biliary obstruction. However, sometimes local resection is done in symptomless patients, despite the absence of any prospective data comparing systematic treatment with the local treatment. Typically, the local treatment includes hepatic resection and stereotactic body radiation therapy to ablate liver metastasis after evaluating the patient carefully. For peripheral lesions more than 5 cm, surgery can be sometimes useful in preserving the liver function than stereotactic body radiation therapy. However, for central lesions, stereotactic body radiation therapy may work better. Further, the retrospective studies suggest local efficacy of radiofrequency ablation, mainly for hepatic lesions that are solitary and smaller than 3cm, not close to the diaphragm, biliary structure, and major vessels. 46–48 The findings of the systematic review of 19 retrospective studies (n = 535 patients) where hepatectomy was performed for metastatic breast cancer. 49 The results showed that median overall survival was 40 months, five-year survival was 40%, and postoperative mortality was 0% to 6%. 49 However, in a subsequent case-control study of 167 patients, overall survival between those who received surgery and/or ablation was not different from those who were treated medically. The findings indicated that local treatment may offer a significant time of disease-free survival and time from systematic chemotherapy among patients with favorable risk-disease. 50
Lastly, the gastrointestinal tract is usually less likely affected by metastasis, and organs such as the stomach and colon are involved followed by the esophagus and small intestine. 51 The choice of local procedure in case of gastrointestinal tract metastasis should be done with the multidisciplinary approach after reviewing the patient’s symptoms, clinical indications, and ruling out other conditions such as primary tumor of the gastrointestinal tract, lymphoma, or any other benign disease. Similarly, ovaries are also a rare site for breast cancer metastasis. 52 Oophorectomy may provide a beneficial therapeutic effect for premenopausal patients with hormone receptor-positive breast cancer irrespective of metastatic disease to the ovaries is present or not. There are limited data available regarding the local management of ovarian metastasis. In a series of 147 patients with ovarian metastasis, overall median survival was 41 months following ovarian metastasectomy. 53
This narrative review provides insights into the role of breast surgery in metastatic breast cancer by synthesizing findings from both observational and interventional studies. The findings from the review of observational studies the beneficial effects of breast surgery in improving the survival of women diagnosed with metastatic breast cancer. These findings are consistent across all retrospective studies included in the narrative review with variation in statistical significance, which may be due to differences in the sample size. Despite the consistent evidence from observational studies, critics consider this as low quality of evidence because of substantial bias associated with observational studies. Regardless of this criticism, a possible explanation for such findings or mechanism by which breast surgery may work is because the primary breast tumor is perhaps a reservoir of tumor cells and can be a source of more cells that can grow and dislodge to other tissues. 54 Therefore, removal of primary breast tumor may reduce the probability of generating new metastasis, thereby improving survival. Besides, the tumor cells may produce growth factors, which can trigger signals that may help the growth of metastatic sites. 55 The evidence from animal studies suggests that resection of the primary tumor may result in a decline in the growth of metastatic tumors. 56
Contrary to this, the evidence from very few RCTs is limited and inconclusive. The findings of the RCTs reveal that breast surgery may improve the two-year survival, local progression-free survival but may not improve the distant progression-free survival and quality of life. Thus, without robust evidence drawn from several RCTs, it is hard to make definite conclusions on the benefits and risks of breast cancer surgery for metastatic breast cancer. Also, the existing evidence is clueless about the breast-specific survival and evidence for toxicity from local treatment does not differ across two groups that only comes from one RCT. Further, women diagnosed with metastatic breast cancer are not a homogenous group of patients rather they differ from each other based on prognoses. It is possible that women with minimal metastasis may have better survival than women whose multiple organs are affected by metastasis. 24 This suggests that RCTs need to be conducted from different countries rather than relying on data from one or a few countries. This is because women in developed countries may be diagnosed at an earlier stage with minimal metastasis, whereas women from developing countries may be diagnosed with multiple organ metastasis. This may affect the results of surgery in two groups of women, therefore, generalizing findings from a few RCTs to all women may not be warranted.
Summary of the Current Guidelines (National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO) in Metastatic Breast Cancer
Survival of patients diagnosed with metastatic breast cancer is increasing as a result of new treatment modalities; therefore, patients should be given optimal treatment to control their symptoms and provide spiritual and psychological support. 57 Typically, such patients are treated with systematic therapy, but this can be given along with locoregional surgery after evaluating the disease status of the patients. Generally, the guidelines recommend taking a multidisciplinary approach for the adequate management of metastatic breast cancer. According to ESMO clinical practice guidelines, treatment decisions of these patients should not depend upon the patient’s age; however, patients’ comorbid, and preferences need to be taken into account by taking a joint multidisciplinary approach. 58 Besides, supportive care should be considered in the overall treatment plan of ABC along with palliative care to control the symptoms of patients. 58 Therapeutic decisions need to be made in a multidisciplinary context for patients who are newly diagnosed with stage IV breast cancer with intact primary tumor. In the absence of symptomatic local disease, locoregional treatment of the primary tumor is not beneficial to improve overall survival and therefore is not recommended in routine. 58 Among patients experiencing local symptoms due to primary tumor or metastasis, there is a need to evaluate the use of local treatment approaches. 58 The resection of the primary tumor may be considered for those patients who are diagnosed with metastasis to bones only, HER2-negative tumors, patients younger than 55 years of age, patients who are diagnosed with HR-positive tumors, patients with oligometastatic disease, and those who respond well to initial systematic therapy. 58 Further, for patients diagnosed with advanced breast cancer (ABC), this includes both inoperable locally advanced breast cancer and metastatic breast cancer. 59 The ABC guidelines, jointly developed by ESMO and The European School of Oncology (ESO), are endorsed by multiple international organizations and have been recently updated with the details of treatment modalities after assessing the symptoms, pathology reports, and other workups of the patient. 59 These guidelines are based on the principle of equal access to multidisciplinary and specialized care including anticancer care, palliative and end-of-life care with full implementation of guidelines tailored to the needs of the patients. 59
Conclusion and Implications for Future Research
The observational studies suggest the beneficial effect of breast surgery in metastatic breast cancer; however, the evidence from RCTs is very limited and inconclusive. Without having firm evidence from RCTs, it is hard to make any conclusions about the benefits of breast surgery because observational studies are usually affected by issues of selection bias and unmeasured confounding. Until the data from large ongoing RCTs is obtained, the decision to perform surgery on a woman diagnosed with metastatic breast cancer should be individualized. Both patient and physician need to decide together about the treatment keeping the risks, benefits, and costs of each intervention in mind. Due to a dearth of robust evidence, future RCTs with larger sample sizes should be carried out both in developing and developed countries, and these RCTs should separately analyze the group of women who are symptomatic versus non-symptomatic or they are provided with systematic therapy or not before the surgery. Also, one needs to consider HER2-positive breast cancer with metastasis and compare the outcomes separately for those who either did or did not obtain anti-HER2 treatment.
Funding Statement
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through large group Research Project under grant number RGP2/263/44.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to it not directly involving human subjects (secondary research study).
Informed Consent Statement
The study itself was a review article and did not involve human subjects and hence patient consent was waived.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
The authors declare no conflicts of interest in this work.
- 1. Parks R, Derks M, Bastiaannet E, Cheung K. Breast cancer epidemiology. In: Breast Cancer Management for Surgeons . Springer; 2018:19–29. [ Google Scholar ]
- 2. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin . 2014;64(1):9–29. doi: 10.3322/caac.21208 [ DOI ] [ PubMed ] [ Google Scholar ]
- 3. DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin . 2016;66(1):31–42. doi: 10.3322/caac.21320 [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer . 2015;136(5):E359–E86. doi: 10.1002/ijc.29210 [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Robb GL, Hortobagyi GN. Advanced therapy of breast disease: PMPH-USA; 2004.
- 6. Khan SA, Stewart AK, Morrow M. Does aggressive local therapy improve survival in metastatic breast cancer? Surgery . 2002;132(4):620–6; discussion 6–7. doi: 10.1067/msy.2002.127544 [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Ly BH, Nguyen NP, Vinh-Hung V, Rapiti E, Vlastos G. Loco-regional treatment in metastatic breast cancer patients: is there a survival benefit? Breast Cancer Res Treat . 2010;119(3):537–545. doi: 10.1007/s10549-009-0610-z [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Giordano SH, Buzdar AU, Smith TL, Kau SW, Yang Y, Hortobagyi GN. Is breast cancer survival improving? Cancer . 2004;100(1):44–52. doi: 10.1002/cncr.11859 [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Thomas A, Khan SA, Chrischilles EA, Schroeder MC. Initial surgery and survival in stage IV breast cancer in the United States, 1988–2011. JAMA Surg . 2016;151(5):424–431. doi: 10.1001/jamasurg.2015.4539 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Sales C, Paiva L, Scandiuzzi D, Anjos AC. Quality of life of breast cancer survivors: social functioning. Rev Bras Cancerol . 2011;47(3):263–272. doi: 10.32635/2176-9745.RBC.2001v47n3.2304 [ DOI ] [ Google Scholar ]
- 11. Wang R, Zhu Y, Liu X, Liao X, He J, Niu L. The clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer . 2019;19(1):1091. doi: 10.1186/s12885-019-6311-z [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Ruiterkamp J, Ernst MFJ. The role of surgery in metastatic breast cancer. Eur J Cancer . 2011;47:S6–S22. doi: 10.1016/S0959-8049(11)70142-3 [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Poscia A, Collamati A, Milovanovic S, et al. Methodological issues in the observational studies conducted in older population: a narrative review. Epidemiol Biostat Public Health . 2017;14(2):e12627–e. [ Google Scholar ]
- 14. Babiera GV, Rao R, Feng L, et al. Effect of primary tumor extirpation in breast cancer patients who present with stage IV disease and an intact primary tumor. Ann Surg Oncol . 2006;13(6):776–782. doi: 10.1245/ASO.2006.03.033 [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Rapiti E, Verkooijen HM, Vlastos G, et al. Complete excision of primary breast tumor improves survival of patients with metastatic breast cancer at diagnosis. J Clin Oncol . 2006;24(18):2743–2749. doi: 10.1200/JCO.2005.04.2226 [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Fields RC, Jeffe DB, Trinkaus K, et al. Surgical resection of the primary tumor is associated with increased long-term survival in patients with stage IV breast cancer after controlling for site of metastasis. Ann Surg Oncol . 2007;14(12):3345–3351. doi: 10.1245/s10434-007-9527-0 [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Gnerlich J, Jeffe DB, Deshpande AD, Beers C, Zander C, Margenthaler JA. Surgical removal of the primary tumor increases overall survival in patients with metastatic breast cancer: analysis of the 1988–2003 SEER data. Ann Surg Oncol . 2007;14(8):2187–2194. doi: 10.1245/s10434-007-9438-0 [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Blanchard DK, Shetty PB, Hilsenbeck SG, Elledge RM. Association of surgery with improved survival in stage IV breast cancer patients. Ann Surg . 2008;247(5):732–738. doi: 10.1097/SLA.0b013e3181656d32 [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Neuman HB, Morrogh M, Gonen M, Van ZKJ, Morrow M, King TA. Stage IV breast cancer in the era of targeted therapy: does surgery of the primary tumor matter? Cancer . 2010;116(5):1226–1233. doi: 10.1002/cncr.24873 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Ruiterkamp J, Ernst M, Van de Poll-Franse L, Bosscha K, Tjan-Heijnen V, Voogd A. Surgical resection of the primary tumour is associated with improved survival in patients with distant metastatic breast cancer at diagnosis. Eur J Surg Oncol . 2009;35(11):1146–1151. doi: 10.1016/j.ejso.2009.03.012 [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Cady B, Nathan N, Michaelson J, Golshan M, Smith B. Matched pair analyses of stage IV breast cancer with or without resection of primary breast site. Ann Surg Oncol . 2008;15(12):3384–3395. doi: 10.1245/s10434-008-0085-x [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Leung AM, Vu HN, Nguyen K-A, Thacker LR, Bear HD. Effects of surgical excision on survival of patients with stage IV breast cancer. J Surg Res . 2010;161(1):83–88. doi: 10.1016/j.jss.2008.12.030 [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. Soran A, Ozmen V, Ozbas S, et al. A randomized controlled trial evaluating resection of the primary breast tumor in women presenting with de novo stage IV breast cancer: Turkish Study (protocol MF07-01). Am J Clin Oncol . 2016;34:1005. doi: 10.1200/JCO.2016.34.15_suppl.1005 [ DOI ] [ Google Scholar ]
- 24. Badwe R, Hawaldar R, Nair N, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol . 2015;16(13):1380–1388. doi: 10.1016/s1470-2045(15)00135-7 [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Bjelic-Radisic V, Fitzal F, Knauer M, et al. Primary surgery versus no surgery in synchronous metastatic breast cancer: patient-reported quality-of-life outcomes of the prospective randomized multicenter ABCSG-28 positive trial. BMC Cancer . 2020;20(1):392. doi: 10.1186/s12885-020-06894-2 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Khan SA, Zhao F, Solin LJ, et al. A randomized Phase III trial of systemic therapy plus early local therapy versus systemic therapy alone in women with de novo stage IV breast cancer: a trial of the ECOG-ACRIN research group (E2108). Am J Clin Oncol . 2020;38:LBA2–LBA2. doi: 10.1200/JCO.2020.38.18_suppl.LBA2 [ DOI ] [ Google Scholar ]
- 27. Loeffler JS, Wen P. Epidemiology, Clinical Manifestations, and Diagnosis of Brain Metastases . Waltham, MA: Google Scholar; 2018. [ Google Scholar ]
- 28. Loeffler JS, Wen P. Overview of the Treatment of Brain Metastases . Waltham, MA: Wolters Kluwer; 2020. [ Google Scholar ]
- 29. Wiegel T, Kreusel KM, Bornfeld N, et al. Frequency of asymptomatic choroidal metastasis in patients with disseminated breast cancer: results of a prospective screening programme. Br J Ophthalmol . 1998;82(10):1159–1161. doi: 10.1136/bjo.82.10.1159 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 30. Mewis L, Young SE. Breast carcinoma metastatic to the choroid. Analysis of 67 patients. Ophthalmology . 1982;89(2):147–151. doi: 10.1016/s0161-6420(82)34838-1 [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Georgalas I, Paraskevopoulos T, Koutsandrea C, et al. Ophthalmic metastasis of breast cancer and ocular side effects from breast cancer treatment and management: mini review. Biomed Res Int . 2015;2015:574086. doi: 10.1155/2015/574086 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Wiegel T, Bottke D, Kreusel KM, et al. External beam radiotherapy of choroidal metastases--final results of a prospective study of the German Cancer Society (ARO 95-08). Radiother Oncol . 2002;64(1):13–18. doi: 10.1016/s0167-8140(02)00134-2 [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Mathis T, Jardel P, Loria O, et al. New concepts in the diagnosis and management of choroidal metastases. Prog Retin Eye Res . 2019;68:144–176. doi: 10.1016/j.preteyeres.2018.09.003 [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Lutz S, Balboni T, Jones J, et al. Palliative radiation therapy for bone metastases: update of an ASTRO evidence-based guideline. Pract Radiat Oncol . 2017;7(1):4–12. doi: 10.1016/j.prro.2016.08.001 [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Chow R, Hoskin P, Schild SE, et al. Single vs multiple fraction palliative radiation therapy for bone metastases: cumulative meta-analysis. Radiother Oncol . 2019;141:56–61. doi: 10.1016/j.radonc.2019.06.037 [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Staren ED, Salerno C, Rongione A, Witt TR, Faber LP. Pulmonary resection for metastatic breast cancer. Arch Surg . 1992;127(11):1282–1284. doi: 10.1001/archsurg.1992.01420110024006 [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Cahan WG, Castro EB. Significance of a solitary lung shadow in patients with breast cancer. Ann Surg . 1975;181(2):137–143. doi: 10.1097/00000658-197502000-00002 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Casey JJ, Stempel BG, Scanlon EF, Fry WA. The solitary pulmonary nodule in the patient with breast cancer. Surgery . 1984;96(4):801–805. [ PubMed ] [ Google Scholar ]
- 39. Friedel G, Pastorino U, Ginsberg RJ, et al. Results of lung metastasectomy from breast cancer: prognostic criteria on the basis of 467 cases of the international registry of lung metastases. Eur J Cardiothorac Surg . 2002;22(3):335–344. doi: 10.1016/s1010-7940(02)00331-7 [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Planchard D, Soria JC, Michiels S, et al. Uncertain benefit from surgery in patients with lung metastases from breast carcinoma. Cancer . 2004;100(1):28–35. doi: 10.1002/cncr.11881 [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Yoshimoto M, Tada K, Nishimura S, et al. Favourable long-term results after surgical removal of lung metastases of breast cancer. Breast Cancer Res Treat . 2008;110(3):485–491. doi: 10.1007/s10549-007-9747-9 [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Meimarakis G, Rüttinger D, Stemmler J, et al. Prolonged overall survival after pulmonary metastasectomy in patients with breast cancer. Ann Thorac Surg . 2013;95(4):1170–1180. doi: 10.1016/j.athoracsur.2012.11.043 [ DOI ] [ PubMed ] [ Google Scholar ]
- 43. Fan J, Chen D, Du H, Shen C, Che G. Prognostic factors for resection of isolated pulmonary metastases in breast cancer patients: a systematic review and meta-analysis. J Thorac Dis . 2015;7(8):1441–1451. doi: 10.3978/j.issn.2072-1439.2015.08.10 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Hoe AL, Royle GT, Taylor I. Breast liver metastases--incidence, diagnosis and outcome. J R Soc Med . 1991;84(12):714–716. doi: 10.1177/014107689108401207 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Atalay G, Biganzoli L, Renard F, et al. Clinical outcome of breast cancer patients with liver metastases alone in the anthracycline-taxane era: a retrospective analysis of two prospective, randomised metastatic breast cancer trials. Eur J Cancer . 2003;39(17):2439–2449. doi: 10.1016/s0959-8049(03)00601-4 [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Veltri A, Gazzera C, Barrera M, et al. Radiofrequency thermal ablation (RFA) of hepatic metastases (METS) from breast cancer (BC): an adjunctive tool in the multimodal treatment of advanced disease. Radiol Med . 2014;119(5):327–333. doi: 10.1007/s11547-013-0354-z [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Pawlik TM, Izzo F, Cohen DS, Morris JS, Curley SA. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol . 2003;10(9):1059–1069. doi: 10.1245/aso.2003.03.026 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Livraghi T, Goldberg SN, Solbiati L, Meloni F, Ierace T, Gazelle GS. Percutaneous radio-frequency ablation of liver metastases from breast cancer: initial experience in 24 patients. Radiology . 2001;220(1):145–149. doi: 10.1148/radiology.220.1.r01jl01145 [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Chua TC, Saxena A, Liauw W, Chu F, Morris DL. Hepatic resection for metastatic breast cancer: a systematic review. Eur J Cancer . 2011;47(15):2282–2290. doi: 10.1016/j.ejca.2011.06.024 [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Sadot E, Lee SY, Sofocleous CT, et al. Hepatic resection or ablation for isolated breast cancer liver metastasis: a case-control study with comparison to medically treated patients. Ann Surg . 2016;264(1):147–154. doi: 10.1097/sla.0000000000001371 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Washington K, McDonagh D. Secondary tumors of the gastrointestinal tract: surgical pathologic findings and comparison with autopsy survey. Mod Pathol . 1995;8(4):427–433. [ PubMed ] [ Google Scholar ]
- 52. Quan ML, Fey J, Eitan R, et al. Role of laparoscopy in the evaluation of the adnexa in patients with stage IV breast cancer. Gynecol Oncol . 2004;92(1):327–330. doi: 10.1016/j.ygyno.2003.10.026 [ DOI ] [ PubMed ] [ Google Scholar ]
- 53. Li W, Wang H, Wang J, L VF, Zhu X, Wang Z. Ovarian metastases resection from extragenital primary sites: outcome and prognostic factor analysis of 147 patients. BMC Cancer . 2012;12:278. doi: 10.1186/1471-2407-12-278 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Bland KI, Copeland EM, Klimberg VS, Gradishar WJ. The Breast E-Book: Comprehensive Management of Benign and Malignant Diseases . Elsevier Health Sciences; 2017. [ Google Scholar ]
- 55. Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature . 2007;449(7162):557–563. doi: 10.1038/nature06188 [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res . 2004;64(6):2205–2211. doi: 10.1158/0008-5472.can-03-2646 [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Caswell-Jin JL, Plevritis SK, Tian L, et al. Change in survival in metastatic breast cancer with treatment advances: meta-analysis and systematic review. JNCI Cancer Spectr . 2018;2(4):pky062. doi: 10.1093/jncics/pky062 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. Gennari A, André F, Barrios CH, et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol . 2021;32(12):1475–1495. doi: 10.1016/j.annonc.2021.09.019 [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol . 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (438.9 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Intensity of metastasis screening and survival outcomes in patients with breast cancer
Jong-ho cheun, jigwang jung, eun-shin lee, jiyoung rhu, han-byoel lee, kyung-hun lee, tae-yong kim, wonshink han, seock-ah im, dong-young noh, hyeong-gon moon.
- Author information
- Article notes
- Copyright and License information
Corresponding author.
Contributed equally.
Received 2020 Jun 2; Accepted 2021 Jan 11; Collection date 2021.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Previous randomized trials, performed decades ago, showed no survival benefit of intensive screening for distant metastasis in breast cancer. However, recent improvements in targeted therapies and diagnostic accuracy of imaging have again raised the question of the clinical benefit of screening for distant metastasis. Therefore, we investigated the association between the use of modern imaging and survival of patients with breast cancer who eventually developed distant metastasis. We retrospectively reviewed data of 398 patients who developed distant metastasis after their initial curative treatment between January 2000 and December 2015. Patients in the less-intensive surveillance group (LSG) had significantly longer relapse-free survival than did patients in the intensive surveillance group (ISG) (8.7 vs. 22.8 months; p = 0.002). While the ISG showed worse overall survival than the LSG did (50.2 vs. 59.9 months; p = 0.015), the difference was insignificant after adjusting for other prognostic factors. Among the 225 asymptomatic patients whose metastases were detected on imaging, the intensity of screening did not affect overall survival. A small subgroup of patients showed poor survival outcomes when they underwent intensive screening. Patients with HR-/HER2 + tumors and patients who developed lung metastasis in the LSG had better overall survival than those in the ISG did. Highly intensive screening for distant metastasis in disease-free patients with breast cancer was not associated with significant survival benefits, despite the recent improvements in therapeutic options and diagnostic techniques.
Subject terms: Breast cancer, Metastasis
Introduction
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer-related death among women 1 . Despite improved overall survival among patients with breast cancer 2 , a significant number of patients eventually develop distant metastasis after initial treatment 3 . The diagnosis of distant metastasis in patients with breast cancer is clinically and psychologically important because the presence of metastasis results in a shift of disease-free status into incurable stage IV status.
Current major guidelines recommend against the use of routine imaging to detect distant metastasis in asymptomatic patients with breast cancer 4 , 5 . These recommendations are based on the findings of randomized trials that showed no survival or quality-of-life benefits on routine intensive imaging studies for breast cancer 6 – 8 . A recently updated systematic review of the randomized trials showed that regular physical examination and yearly mammograms are as effective as highly intensive imaging considering overall survival 9 . Thus, intensive screening for distant metastasis does not provide survival benefit but rather increases the risk of extending the duration of toxic treatment, as intensive screening might result in the earlier detection of metastatic lesions 6 , 7 .
However, the above-mentioned randomized trials were conducted nearly three decades ago when treatment strategies for resectable breast cancer were substantially different from those used currently. Moreover, the survival of patients with metastatic breast cancer has significantly improved over the last three decades 10 – 12 . Furthermore, a subset of patients with metastatic breast cancer experience durable clinical remission when they are treated with intensive multidisciplinary approaches for oligometastatic lesions 13 , 14 . Finally, there has been a significant improvement in the diagnostic accuracy of various imaging techniques. Thus, the clinical benefit of intensive screening for distant metastasis should be reevaluated.
Retrospective analysis of the benefit of intensive screening for patients with breast cancer has major drawbacks: patients at a higher risk of developing distant metastasis may undergo imaging tests more frequently, resulting in selection bias 15 , 16 . Moreover, patients who undergo intensive screening may show improved post-relapse survival, as the metastatic lesions might be detected earlier, resulting in lead-time bias, and the lesions can be biologically indolent, causing length bias 17 . Accordingly, in the present study of 398 patients with breast cancer with distant metastasis, we tried to minimize selection bias by excluding all patients without distant metastasis and aimed to negate lead-time bias by defining survival as the duration between the date of initial treatment and the date of death.
Patients and methods
We obtained the baseline clinical data and reviewed the detailed information of patients with breast cancer who were diagnosed between January 2000 and July 2015 from our institutional database of patients with breast cancer. We included patients who developed distant metastasis after the initial recurrence-free survival (RFS) treatment. We excluded patients with synchronous or metachronous malignancies in other organs, bilateral breast cancer, male breast cancer, and recurrent breast cancer. We identified 398 patients who were initially diagnosed with non-metastatic, resectable breast cancer, received follow-up care in our institution, and eventually developed distant metastasis. From the database, we obtained the baseline characteristics and clinicopathologic information. Initial breast cancer was pathologically staged according to the 7th AJCC criteria. Hormone receptor (i.e., HR, including estrogen and/or progesterone receptors) data were collected according to immunohistochemistry findings, with positivity defined as > 1%. Human epidermal growth factor receptor type 2 (HER2) status was evaluated with anti-HER2 antibodies and/or fluorescence in situ hybridization. We also collected the data regarding the use of various imaging studies including chest radiography, bone scintigraphy, computed tomography (CT), ultrasonography (USG), magnetic resonance imaging (MRI), and fludeoxyglucose-positron emission tomography ( 18 F-FDG/PET).
Distant metastasis and screening intensity
Distant metastasis was defined as any recurrences at any sites outside the breast and regional lymph nodes. The metastatic sites included the bones, lungs, pleura, liver, brain, and distant lymph nodes; they were classified into bone, visceral (lung, pleura, liver, brain, and distant lymph node), and mixed metastases (bone and visceral) for comparison. When metastases were observed in multiple organs within 2 months of treatment, they were defined as multiple site metastases. The clinical diagnosis of distant metastasis was made after histologic confirmation of metastasis or imaging findings compatible with metastasis when biopsy was not feasible. We also reviewed the presence of symptoms associated with metastases using each patient’s medical records. Ambiguous cases such as the perception of symptoms after knowing the presence of metastasis or symptoms not associated with the site of metastases were considered asymptomatic.
To assess the intensity of distant metastasis screening, we calculated the time interval between the date of clinical diagnosis of distant metastasis and the date of previous imaging examinations that targeted the organ where the metastasis developed. For example, if a patient developed bone metastasis, the screening intensity was determined considering the date of the previous bone scintigraphy or 18 F-FDG/PET. For lung and liver metastases, the dates of chest radiography, chest CT, and 18F-FDG/PET and the dates of abdominal USG, abdominal CT, and 18 F-FDG/PET were considered, respectively (Fig. 1 ). Additionally, we performed same analysis using a different definition of surveillance intensity by calculating total number of exams between the time of operation and diagnosis of distant metastasis dividing with RFS for each patient. Moreover, only tests that were conducted within 2 years before the occurrence of metastasis were analyzed separately.
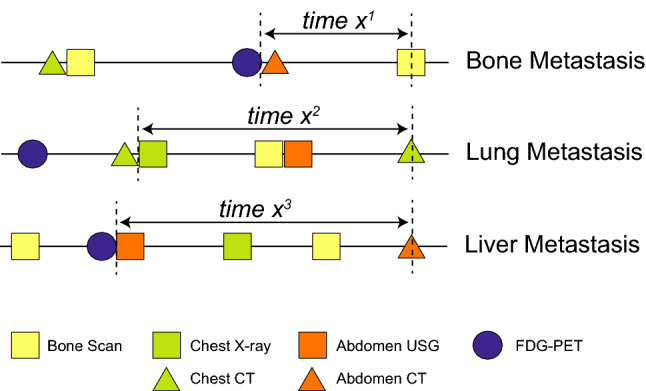
Definition of time intervals. We calculated the interval between the date of clinical diagnosis of metastasis and the date of previous imaging studies of target organs. For instance, for a patient with lung metastasis, as shown in this figure, the time interval (X 2 ) was defined as the date between chest CT at diagnosis of metastasis and previous chest radiography, not including abdominal USG and abdominal CT. 18 F-FDG-PET was allowed regardless of metastasis sites. The time intervals for patients with bone metastasis (X 1 ) and those with liver metastasis (X 3 ) were calculated with the same principles. USG, ultrasonography; CT, computed tomography; 18 F-FDG-PET, fludeoxyglucose-positron emission tomography.
Statistical analyses
Overall survival was the time between the date of initial diagnosis and the date of death. Recurrence-free survival was the time from the date of initial diagnosis to the date of first clinical diagnosis of distant metastasis. The date of death was obtained from the Office for National Statistics of Korea. Survival analyses were performed with the Kaplan–Meier method. The log-rank test and Gehan-Breslow-Wilcoxon test were used to compare survival curves. The Cox proportional hazards regression model was used for multivariate survival analysis. Variables that showed a P -value < 0.05 on the log-rank or Breslow test were included in multivariate analysis. All analyses were performed using SPSS (version 22.0; SPSS, Inc.). The statistical significance was set at P < 0.05.
Ethics approval and consent to participate
This study was approved by the institutional review board (IRB) of Seoul National University Hospital (SNUH; IRB No. H-1905-047-1031), and the study followed the Declaration of Helsinki and good clinical practice guidelines. All patients gave informed consent.
Patient characteristics
We identified 398 patients with breast cancer who developed distant metastasis and who met the inclusion criteria. The mean age at the time of initial treatment was 47.6 ± 11.0 years. Almost half of the patients had stage III breast cancer initially (45.8%), and two-thirds of the patients underwent mastectomy (68.6%). The clinical characteristics of the included patients are listed in Table 1 . The interval between the detection of metastatic lesions and the date of previous imaging studies for the particular organ for each individual patient is shown in Fig. 1 . The median interval between the previous imaging study and the detection of metastasis was 10.5 ± 9.8 months. Our patients were classified into two groups: the intensive screening group (ISG, n = 199) and the less-intensive screening group (LSG, n = 199), with median intervals of 4.5 ± 1.6 and 16.4 ± 11.0 months, respectively. The ISG had a significantly higher incidence of neoadjuvant chemotherapy, postoperative radiotherapy, stage III disease, and previous history of locoregional recurrence, and was more likely to be diagnosed in more recent years (Table 1 ).
Demographic and clinical characteristics of patients.
Data are number of patients and percent (%) or mean ± standard deviation.
ISG intensive surveillance group, LSG less-intensive surveillance group, BMI body mass index, BCS breast-conserving surgery, HER2 human epidermal growth factor receptor 2, TNBC triple-negative breast cancer.
a Stratified according to the American Joint Committee on Cancer (AJCC) 7 th TNM stage.
Survival outcomes and screening intensity
The distant-metastasis free survival of the 398 patients with breast cancer according to the frequency of imaging studies is shown in Fig. 2 a. Patients in the ISG had a significantly shorter distant-metastasis free survival especially in the early phase of follow-up than in the LSG (log rank p = 0.083, Breslow p = 0.002). The LSG had a significantly higher overall survival (log rank p = 0.046, Breslow p = 0.015, Fig. 2 b). However, after adjusting for other prognostic factors, multivariate Cox regression analysis revealed no significant difference in overall survival between the two groups (hazard ratio [HR] = 1.21, 95% confidence interval [CI]: 0.95–1.54; p = 0.124; Table 2 ). The initial N stage, hormone receptor status, Ki-67 expression level, history of previous locoregional recurrence, presence of symptoms at the diagnosis of distant metastasis, and metastatic site remained independent factors predicting overall survival. Additionally, we used different definition of surveillance intensity based on the number of all exams during the RFS. The survival analysis showed similar results including that of the Cox-regression analysis (Supplementary Fig. 1 ). To further minimize the effect of the confounding variables, we conducted propensity score matching incorporating initial N stage, hormone receptor status, Ki-67 expression level, history of previous locoregional recurrence, presence of symptoms and metastatic sites. Based on propensity score matching, a total of 159 pairs of patients was included in the survival analysis. There was no difference in overall survival between matched LSG and ISG groups (log rank p = 0.264, Breslow p = 0.129, Supplementary Fig. 2 a).
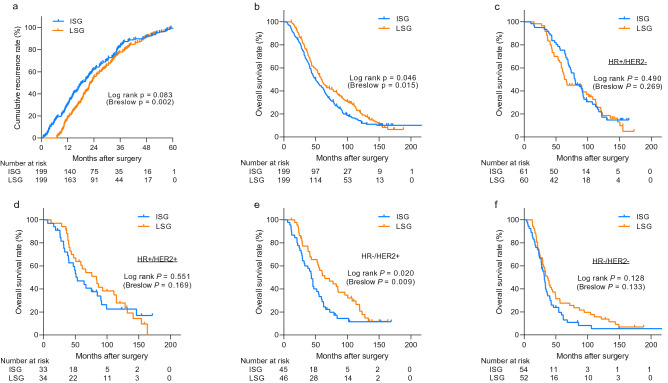
Kaplan–Meier curves showing recurrence-free survival and overall survival of all patients. The Kaplan–Meier curves show recurrence-free survival (a) and overall survival (b–f) . The survival curves for all 398 patients (b) and for patients stratified according to the hormone receptor and HER2-overexpression status (c–f) are shown. Among all, 13 patients were unable to obtain the data of subtypes. P-values were calculated by using the log-rank test along with Gehan–Breslow–Wilcoxon tests (in the parenthesis). ISG intensive surveillance group, LSG less-intensive surveillance group, HR hormone receptor, HER2 human epidermal growth factor receptor type 2.
Clinicopathologic features affecting post-operative overall survival.
HR hazard ratio, CI confidence interval, BMI body mass index, HER2 human epidermal growth factor receptor 2, Ref. reference.
*The p values are derived from the Log-rank test and the p values from the Breslow test are shown in the parenthesis.
We then examined the association between the screening intensity for distant metastasis and survival considering different subtypes of breast cancer. There was no significant interaction between variables including subtypes and sites of metastasis and intensity of surveillance (p > 0.05, data not shown). As shown in Fig. 2 c–f and supplementary Fig. 3 , the screening intensity did not affect the survival outcomes considering HR + /HER2-, HR + /HER2 + , and HR-/HER2- subtypes. However, the LSG group had significantly better overall survival than the ISG group did when the tumors were HR-/HER2 + . Nevertheless, the prognostic importance of screening intensity did not remain significant in this subgroup after adjusting for other prognostic factors by using a Cox regression model (HR = 1.47, 95% CI: 0.80–2.73; p = 0.217, Supplement Table 1 ).
Sites of metastasis, presence of symptoms, and effects of screening intensity
Among the 398 patients with distant metastasis, 220 developed distant metastasis in a single organ: 100 patients had bone metastasis, 85 had lung metastasis, and 35 had liver metastasis. The remaining 178 patients developed metastases in multiple organs. The intensity of screening did not affect the overall survival of patients who developed metastasis in the bones, liver, or multiple organs. However, the overall survival of patients whose first site of metastasis was the lungs was significantly low (Fig. 3 ). The screening intensity remained an independent prognostic factor of overall survival in patients with lung metastasis after adjusting for other prognostic factors (HR = 2.10, 95% CI: 1.06–4.17; p = 0.034, Supplement Table 2 ). However, the patients with lung metastasis in the ISG showed higher incidence of symptoms (43.9% vs. 19.5%, p = 0.018) and previous history of local recurrences (41.5% vs. 14.6%, p = 0.007).
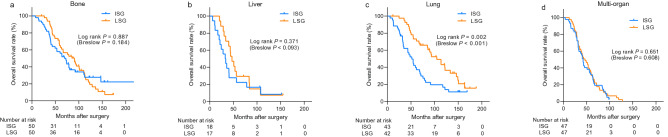
Kaplan–Meier curves showing overall survival depending on initial metastases sites. The survival curves for patients with bone (a) , liver (b) and multiple metastases (d) showed no significant differences between the two groups. However, the intensive surveillance group showed a significantly low overall survival among patients with lung metastasis (c) . ISG intensive surveillance group, LSG less-intensive surveillance group.
As the presence of symptoms at the time of diagnosis might lead to the performance of imaging studies earlier than the pre-scheduled dates, patients who develop symptomatic, rapidly progressing distant metastasis are more likely to have a shorter time interval between the previous imaging studies and the diagnosis of distant metastasis. To overcome this issue, we identified 225 patients whose distant metastases were asymptomatic and who were diagnosed using screening imaging studies. As shown in Fig. 4 a, we observed similar associations between the screening intensity and the survival outcomes of patients with asymptomatic distant metastasis. Also, the propensity score matching analysis for asymptomatic patients using 96 pairs of patients showed the same result (Supplementary Fig. 2 b). Patients in the LSG had significantly higher overall survival when the patients had HR-/HER2 + tumors and when the first site of metastasis was the lungs (Fig. 4 b–h, Supplementary Fig. 3 ). Among these 225 asymptomatic patients, we also observed that the screening intensity showed borderline prognostic significance in HR−/HER2− subtype (Fig. 4 e). However, the interaction assessment among asymptomatic patients revealed significant interaction between both hormone receptor status (p = 0.001) and the metastasis site (p = 0.022) and the intensity of surveillance.
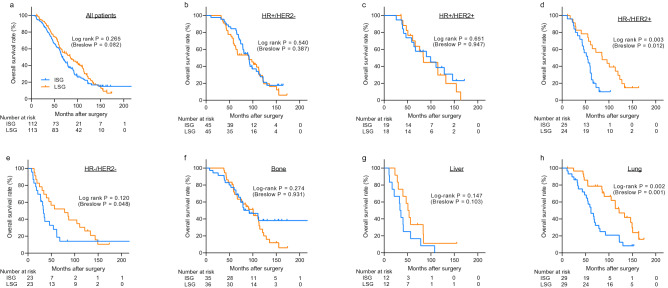
Kaplan–Meier curves showing overall survival of asymptomatic patients and subgroups. The Kaplan–Meier curves show the overall survival of 225 asymptomatic patients. Overall survival was analyzed after dividing the patients into the intensive surveillance group and less-intensive surveillance group (a) . Further subgroup analysis was performed according to subtypes (b–e) and sites of metastases (f–h) . Among asymptomatic patients, 3 patients were unable to obtain the data of subtypes. ISG intensive surveillance group, LSG less-intensive surveillance group, HR hormone receptor, HER2 human epidermal growth factor receptor type 2.
In the current study, we showed that intensive imaging during the post-treatment follow-up period was not associated with survival benefit in patients with breast cancer. We also observed that in a subset of patients, i.e., patients with lung metastasis, highly intensive screening for distant metastasis was associated with poor survival outcomes. Our data indicate that, despite the recent development in targeted therapy for patients with stage IV breast cancer, earlier detection of distant metastasis does not result in survival benefit for patients with breast cancer who developed distant metastasis.
Two randomized controlled trials have evaluated the efficacy of intensity of surveillance in patients with breast cancer. In 1994, the Interdisciplinary Group for Cancer Care Evaluation (GIVIO) 7 randomized 1320 patients with breast cancer into intensive or clinical surveillance; they reported that treatment outcomes and quality of life were not significantly different between the groups after follow-up for 71 months. Similarly, Del Turco et al. and Palli et al. 6 , 18 enrolled 1,243 patients, and showed significantly higher recurrence free survival for clinical groups, but failed to show significant difference on overall mortality at 5- and 10-years of follow-ups. The data showed the lack of survival benefit of intensive surveillance for distant metastasis; these are the basis of the current guidelines that recommend against routine imaging studies—except mammography—for asymptomatic disease-free patients with breast cancer 4 , 5 , 19 . However, these trials were conducted before the era of targeted therapies such as trastuzumab ( NCT00829166 ) or CDK inhibitors ( NCT01740427 ), which have significantly improved the survival of patients with stage IV breast cancer 20 , 21 . Moreover, the diagnostic accuracy of modern imaging studies has substantially improved since these clinical trials 22 – 24 . Therefore, our study included patients who were diagnosed between 2000 and 2015, and the results show that intensive surveillance still lacks any survival benefit.
Although current guidelines 4 , 5 , 19 and systematic review 9 , 25 do not recommend routine imaging for patients with breast cancer, real-word practices often involve the use of advanced imaging studies owing to the belief that earlier detection of distant metastasis may lead to improved survival 15 , 16 , 26 , 27 . Moreover, diagnostic studies for distant metastasis may provide emotional support and reassurance to both the physician and patient 6 , 28 – 30 . However, frequent visits may elevate the anxiety of breast cancer survivors 31 , and intensive surveillance may increase false-positive results for distant metastasis that may further increase the psychological burden 32 , 33 . Furthermore, Meyer et al. 34 reported a significant association between intensive surveillance and the risk of secondary cancer or radiation-induced malignancy in the patient’s lifetime. Therefore, the decision regarding optimal surveillance after the initial treatment for breast cancer must be well-balanced after considering the advantages and disadvantages.
The current study has several limitations. This was a retrospective study from a single, high-volume institution. The retrospective nature inherently raises the possibility of selection bias. To eliminate the effect of selection bias that high-risk patients with breast cancer may undergo very intensive screening, we limited our analysis to patients who eventually developed distant metastasis. Despite our efforts of excluding patients who did not develop distant metastasis, patients with more aggressive features within the study population were more likely to undergo intensive surveillance, as indicated in our results. This selection bias may have masked the potential protective effect of intensive surveillance, because the high-risk features—such as triple-negative subtype or advanced stage at diagnosis—are associated with shorter time to death after the development of distant metastasis 12 . Therefore, our current findings require further validation by using data from a multi-institutional database or a nationwide registry. However, obtaining detailed clinical information about the type of metastasis and the use of imaging studies remain a major hurdle for such validation studies. In addition, we could not adjust for the complex information regarding the use of adjuvant systemic therapies and the response to palliative systemic treatment. Moreover, our study population included a substantial proportion of patients whose disease severity was determined by using the clinical TNM staging because they underwent neoadjuvant therapy. The potential discrepancy between the clinical stage and anatomic stage of breast cancer might have made our results more complex 35 . Finally, it is unclear why intensive surveillance was associated with worse overall survival in patients with HR−/HER2 + tumors or patients who developed lung metastasis in our study. It is possible that small number of patients in each subgroup or unknown confounding factors may have resulted this observation. Additionally, unadjusted confounding factors may have contributed to this observation. Cox-regression analysis also showed significant interaction between both hormone receptor status or metastasis sites and intensive surveillance. Unfortunately, we were not able to perform additional propensity score matching analysis due to the small number of patients in this subgroup. Alternatively, we cannot also exclude the possibility that higher anxiety caused by intensive screening may have affected the survival outcomes, because stress and anxiety promoted tumor progression in mouse models of various solid tumors including breast tumors 36 – 38 .
The results of this retrospective study suggest the lack of any association between intensive surveillance for distant metastasis and survival benefit in asymptomatic, disease-free patients with breast cancer after their initial treatments.

Supplementary Information
Author contributions.
Study concepts and design was done by J.-H.C., J.J. and H.-G.M. Collection and assembly of data were performed by J.-H.C., J.J., E.-S.L. and J.R. Data analysis and interpretation were performed by J.-H.C., J.J., H.-B.L., W.H. and H.-G.M. Statistical analysis was done by J.-H.C., J.J. and H.-G.M. Manuscript preparation and editing was performed by J.-H.C., J.J., and H.-G.M. Manuscript review was done by K.-H.L., T.-Y.K., H.-B.L., W.H., S.-A.I. and D.-Y.N. Final approval of manuscript was performed by all authors.
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HA15C0011). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT, Republic of Korea (MSIT) (No NRF-2019R1A2C2005277).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jong-Ho Cheun and Jigwang Jung.
The online version contains supplementary material available at 10.1038/s41598-021-82485-w.
- 1. Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Allemani C, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Colleoni M, et al. Annual hazard rates of recurrence for breast cancer during 24 years of follow-up: Results from the international breast cancer study group trials I to V. J. Clin. Oncol. 2016;34:927–935. doi: 10.1200/JCO.2015.62.3504. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. Cardoso F, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019 doi: 10.1093/annonc/mdz189. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Runowicz CD, et al. American cancer society/American society of clinical oncology breast cancer survivorship care guideline. J. Clin. Oncol. 2016;34:611–635. doi: 10.1200/JCO.2015.64.3809. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Del Turco MR. Intensive diagnostic follow-up after treatment of primary breast cancer. JAMA. 1994;271:1593. doi: 10.1001/jama.1994.03510440053032. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Ghezzi P. Impact of follow-up testing on survival and health-related quality of life in breast cancer patients. JAMA. 1994;271:1587. doi: 10.1001/jama.1994.03510440047031. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Kokko R, Hakama M, Holl K. Role of chest X-ray in diagnosis of the first breast cancer relapse: A randomized trial. Breast Cancer Res. Treat. 2003;81:33–39. doi: 10.1023/A:1025419114857. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Moschetti I, Cinquini M, Lambertini M, Levaggi A, Liberati A. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst. Rev. 2016;7:150. doi: 10.1002/14651858.CD001768.pub3. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Cardoso F, et al. Global analysis of advanced/metastatic breast cancer: Decade report (2005–2015) Breast. 2018;39:131–138. doi: 10.1016/j.breast.2018.03.002. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Sundquist M, Brudin L, Tejler G. Improved survival in metastatic breast cancer 1985–2016. Breast. 2017;31:46–50. doi: 10.1016/j.breast.2016.10.005. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Lee ES, et al. Identifying the potential long-term survivors among breast cancer patients with distant metastasis. Ann. Oncol. 2016;27:828–833. doi: 10.1093/annonc/mdw036. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Hanrahan EO, et al. Combined-modality treatment for isolated recurrences of breast carcinoma. Cancer. 2005;104:1158–1171. doi: 10.1002/cncr.21305. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Kobayashi T, et al. Possible clinical cure of metastatic breast cancer: Lessons from our 30-year experience with oligometastatic breast cancer patients and literature review. Breast Cancer. 2012;19:218–237. doi: 10.1007/s12282-012-0347-0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Hahn EE, Hays RD, Kahn KL, Litwin MS, Ganz PA. Use of imaging and biomarker tests for posttreatment care of early-stage breast cancer survivors. Cancer. 2013;119:4316–4324. doi: 10.1002/cncr.28363. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Panageas KS, Sima CS, Liberman L, Schrag D. Use of high technology imaging for surveillance of early stage breast cancer. Breast Cancer Res. Treat. 2012;131:663–670. doi: 10.1007/s10549-011-1773-y. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Lawrence G, et al. Population estimates of survival in women with screen-detected and symptomatic breast cancer taking account of lead time and length bias. Breast Cancer Res. Treat. 2009;116:179–185. doi: 10.1007/s10549-008-0100-8. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Palli D. Intensive vs clinical follow-up after treatment of primary breast cancer: 10-year update of a randomized trial. JAMA J. Am. Med. Assoc. 1999;281:1586–1586. doi: 10.1001/jama.281.17.1586. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Network, N. C. C. NCCN Guidelines with NCCN Evidence Blocks. Breast Cancer Version 3.2019 . (2019).
- 20. Verma S, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Finn RS, et al. Palbociclib and letrozole in advanced breast cancer. N. Engl. J. Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Yoon JH, Kim MJ, Kim EK, Moon HJ. Imaging surveillance of patients with breast cancer after primary treatment: Current recommendations. Korean J. Radiol. 2015;16:219–228. doi: 10.3348/kjr.2015.16.2.219. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Murakami R, et al. FDG-PET/CT in the diagnosis of recurrent breast cancer. Acta radiol. 2012;53:12–16. doi: 10.1258/ar.2011.110245. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Engelhard K, Hollenbach HP, Wohlfart K, von Imhoff E, Fellner FA. Comparison of whole-body MRI with automatic moving table technique and bone scintigraphy for screening for bone metastases in patients with breast cancer. Eur. Radiol. 2004;14:99–105. doi: 10.1007/s00330-003-1968-7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Lafranconi A, et al. Intensive follow-up for women with breast cancer: Review of clinical, economic and patient’s preference domains through evidence to decision framework. Health Qual. Life Outcomes. 2017;15:1–18. doi: 10.1186/s12955-017-0779-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 26. Sperduti I, et al. Breast cancer follow-up strategies in randomized phase III adjuvant clinical trials: A systematic review. J. Exp. Clin. Cancer Res. 2013;32:1. doi: 10.1186/1756-9966-32-89. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Kim KS, et al. The reality in the follow-up of breast cancer survivors: Survey of Korean Breast Cancer Society. Ann. Surg. Treat. Res. 2015;88:133. doi: 10.4174/astr.2015.88.3.133. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Van Hezewijk M, et al. Professionals’ opinion on follow-up in breast cancer patients; Perceived purpose and influence of patients’ risk factors. Eur. J. Surg. Oncol. 2011;37:217–224. doi: 10.1016/j.ejso.2011.01.001. [ DOI ] [ PubMed ] [ Google Scholar ]
- 29. Brown L, Payne S, Royle G. Patient initiated follow up of breast cancer. Psychooncology. 2002;11:346–355. doi: 10.1002/pon.576. [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Feiten S, et al. Follow-up reality for breast cancer patients—Standardised survey of patients and physicians and analysis of treatment data. Geburtshilfe Frauenheilkd. 2016;76:557–563. doi: 10.1055/s-0042-106210. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Paradiso A, Nitti P, Frezza P, Scorpiglione N. A survey in Puglia: The attitudes and opinions of specialists, general physicians and patients on follow-up practice. Ann. Oncol. 1995;6:S53–S56. doi: 10.1093/annonc/6.suppl_2.S53. [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Tomiak E, Piccart M. Routine follow-up of patients after primary therapy for early breast cancer: Changing concepts and challenges for the future. Ann. Oncol. 1993;4:199–204. doi: 10.1093/oxfordjournals.annonc.a058456. [ DOI ] [ PubMed ] [ Google Scholar ]
- 33. Rusch P, et al. Distant metastasis detected by routine staging in breast cancer patients participating in the national German screening programme: consequences for clinical practice. Springerplus. 2016;5:1010. doi: 10.1186/s40064-016-2703-6. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Meyer C, et al. Intensive imaging surveillance of survivors of breast cancer may increase risk of radiation-induced malignancy. Clin. Breast Cancer. 2019;19:e468–e474. doi: 10.1016/j.clbc.2019.01.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Abner AL, et al. Correlation of tumor size and axillary lymph node involvement with prognosis in patients with T1 breast carcinoma. Cancer. 1998;83:2502–2508. doi: 10.1002/(SICI)1097-0142(19981215)83:12<2502::AID-CNCR14>3.0.CO;2-I. [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Thaker PH, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006;12:939–944. doi: 10.1038/nm1447. [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Jang H-J, Boo H-J, Lee HJ, Min H-Y, Lee H-Y. Chronic stress facilitates lung tumorigenesis by promoting exocytosis of IGF2 in lung epithelial cells. Cancer Res. 2016;76:6607–6619. doi: 10.1158/0008-5472.CAN-16-0990. [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Hassan S, et al. Behavioral stress accelerates prostate cancer development in mice. J. Clin. Invest. 2013 doi: 10.1172/JCI63324. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data availability statement.
- View on publisher site
- PDF (901.4 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Advertisement
Epigenetic regulation of breast cancer metastasis
- Published: 19 October 2023
- Volume 43 , pages 597–619, ( 2024 )
Cite this article

- Chitra Thakur 1 ,
- Yiran Qiu 1 ,
- Aashna Pawar 1 &
- Fei Chen 1
1302 Accesses
3 Citations
1 Altmetric
Explore all metrics
Breast cancer is the most frequently diagnosed malignancy and the second leading cause of cancer-related mortality among women worldwide. Recurrent metastasis is associated with poor patient outcomes and poses a significant challenge in breast cancer therapies. Cancer cells adapting to a new tissue microenvironment is the key event in distant metastasis development, where the disseminating tumor cells are likely to acquire genetic and epigenetic alterations during the process of metastatic colonization. Despite several decades of research in this field, the exact mechanisms governing metastasis are not fully understood. However, emerging body of evidence indicates that in addition to genetic changes, epigenetic reprogramming of cancer cells and the metastatic niche are paramount toward successful metastasis. Here, we review and discuss the latest knowledge about the salient attributes of metastasis and epigenetic regulation in breast cancer and crucial research domains that need further investigation.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
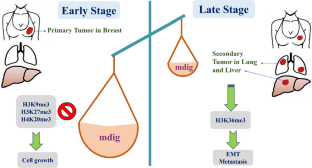
Similar content being viewed by others

Epigenetic Mechanisms of Cancer Metastasis

Tumor Microenvironment and Epigenetic Implications in Breast Cancer Progression

A three layered histone epigenetics in breast cancer metastasis
Data availability, abbreviations.
epidermal growth factor receptor 2
estrogen receptor
progesterone receptor
breast cancer
triple-negative breast cancer
disseminated tumor cells
extracellular matrix
epithelial-mesenchymal transition
mesenchymal-epithelial transition
pre-metastatic niche
extracellular vesicles
vascular endothelial growth factor
matrix metalloproteinases
circulating tumor cells
cancer stem cells
breast cancer stem cells
transcription factors
enhancer of zeste homolog 2
programmed death ligand 1
lysyl oxidase
manganese superoxide dismutase
proline dehydrogenase
cytosine-phosphate-guanine
ADAM metallopeptidase domain 12
urokinase-type plasminogen activator
circulating tumor
histone acetyltransferases
histone methyltransferases
histone deacetylases
histone demethylases
protein arginine methyltransferases
suberanilohydroxamic acid
poly(ADP-ribose) polymerase
Siegel, R. L., et al. (2023). Cancer statistics, 2023. CA: a Cancer Journal for Clinicians, 73 (1), 17–48.
PubMed Google Scholar
Akhtar, M., et al. (2019). Paget’s “seed and soil” theory of cancer metastasis: An idea whose time has come. Advances in Anatomic Pathology, 26 (1), 69–74.
Article CAS PubMed Google Scholar
Chambers, A. F., Groom, A. C., & MacDonald, I. C. (2002). Dissemination and growth of cancer cells in metastatic sites. Nature Reviews. Cancer, 2 (8), 563–572.
Ribatti, D., Mangialardi, G., & Vacca, A. (2006). Stephen Paget and the 'seed and soil' theory of metastatic dissemination. Clinical and Experimental Medicine, 6 (4), 145–149.
Fragomeni, S. M., Sciallis, A., & Jeruss, J. S. (2018). Molecular subtypes and local-regional control of breast cancer. Surgical Oncology Clinics, 27 (1), 95–120.
Jones, R. L., Constantinidou, A., & Reis-Filho, J. S. (2012). Molecular classification of breast cancer. Surgical Pathology, Clinical, 5 (3), 701–717.
Article Google Scholar
Dent, R., et al. (2007). Triple-negative breast cancer: Clinical features and patterns of recurrence. Clinical Cancer Research, 13 (15 Pt 1), 4429–4434.
Article PubMed Google Scholar
André, F., & Zielinski, C. C. (2012). Optimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agents. Annals of Oncology, 23 , vi46.
Shiovitz, S., & Korde, L. A. (2015). Genetics of breast cancer: A topic in evolution. Annals of Oncology, 26 (7), 1291–1299.
Article CAS PubMed PubMed Central Google Scholar
Shahbandi, A., Nguyen, H. D., & Jackson, J. G. (2020). TP53 mutations and outcomes in breast cancer: Reading beyond the headlines. Trends Cancer, 6 (2), 98–110.
Corso, G., et al. (2018). Prognosis and outcome in CDH1-mutant lobular breast cancer. European Journal of Cancer Prevention, 27 (3), 237–238.
Article PubMed PubMed Central Google Scholar
Corso, G., et al. (2016). CDH1 germline mutations and hereditary lobular breast cancer. Familial Cancer, 15 (2), 215–219.
Kechagioglou, P., et al. (2014). Tumor suppressor PTEN in breast cancer: Heterozygosity, mutations and protein expression. Anticancer Research, 34 (3), 1387–1400.
CAS PubMed Google Scholar
Chen, J., & Lindblom, A. (2000). Germline mutation screening of the STK11/LKB1 gene in familial breast cancer with LOH on 19p. Clinical Genetics, 57 (5), 394–397.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: The next generation. Cell, 144 (5), 646–674.
van Denderen, B. J., & Thompson, E. W. (2013). Cancer: The to and fro of tumour spread. Nature, 493 (7433), 487–488.
Chaffer, C. L., & Weinberg, R. A. (2011). A perspective on cancer cell metastasis. Science, 331 (6024), 1559–1564.
Gupta, G. P., & Massagué, J. (2006). Cancer metastasis: Building a framework. Cell, 127 (4), 679–695.
Fares, J., et al. (2020). Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduction and Targeted Therapy, 5 (1), 28.
Klein, C. A. (2009). Parallel progression of primary tumours and metastases. Nature Reviews. Cancer, 9 (4), 302–312.
Jin, X., & Mu, P. (2015). Targeting breast cancer metastasis. Breast Cancer (Auckl.), 9 (Suppl 1), 23–34.
Talmadge, J. E., & Fidler, I. J. (2010). AACR centennial series: The biology of cancer metastasis: historical perspective. Cancer Research, 70 (14), 5649–5669.
Nguyen, D. X., Bos, P. D., & Massagué, J. (2009). Metastasis: From dissemination to organ-specific colonization. Nature Reviews. Cancer, 9 (4), 274–284.
Pantel, K., & Brakenhoff, R. H. (2004). Dissecting the metastatic cascade. Nature Reviews. Cancer, 4 (6), 448–456.
Valastyan, S., & Weinberg, R. A. (2011). Tumor metastasis: Molecular insights and evolving paradigms. Cell, 147 (2), 275–292.
Labelle, M., & Hynes, R. O. (2012). The initial hours of metastasis: The importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discovery, 2 (12), 1091–1099.
Leber, M. F., & Efferth, T. (2009). Molecular principles of cancer invasion and metastasis (review). International Journal of Oncology, 34 (4), 881–895.
Mouw, J. K., Ou, G., & Weaver, V. M. (2014). Extracellular matrix assembly: A multiscale deconstruction. Nature Reviews. Molecular Cell Biology, 15 (12), 771–785.
Cheung, K. J., & Ewald, A. J. (2016). A collective route to metastasis: Seeding by tumor cell clusters. Science, 352 (6282), 167–169.
Friedl, P., & Wolf, K. (2003). Tumour-cell invasion and migration: Diversity and escape mechanisms. Nature Reviews. Cancer, 3 (5), 362–374.
Brabletz, T., et al. (2018). EMT in cancer. Nature Reviews. Cancer, 18 (2), 128–134.
Acloque, H., et al. (2009). Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. The Journal of Clinical Investigation, 119 (6), 1438–1449.
Thiery, J. P., et al. (2009). Epithelial-mesenchymal transitions in development and disease. Cell, 139 (5), 871–890.
Charpentier, M., & Martin, S. (2013). Interplay of stem cell characteristics, EMT, and microtentacles in circulating breast tumor cells. Cancers (Basel), 5 (4), 1545–1565.
Onder, T. T., et al. (2008). Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Research, 68 (10), 3645–3654.
Chao, Y. L., Shepard, C. R., & Wells, A. (2010). Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Molecular Cancer, 9 , 179.
Bonnomet, A., et al. (2012). A dynamic in vivo model of epithelial-to-mesenchymal transitions in circulating tumor cells and metastases of breast cancer. Oncogene, 31 (33), 3741–3753.
Moreno-Bueno, G., Portillo, F., & Cano, A. (2008). Transcriptional regulation of cell polarity in EMT and cancer. Oncogene, 27 (55), 6958–6969.
Si, W., et al. (2015). Dysfunction of the reciprocal feedback loop between GATA3- and ZEB2-nucleated repression programs contributes to breast cancer metastasis. Cancer Cell, 27 (6), 822–836.
Duan, H., et al. (2017). TET1 inhibits EMT of ovarian cancer cells through activating Wnt/β-catenin signaling inhibitors DKK1 and SFRP2. Gynecologic Oncology, 147 (2), 408–417.
Hu, W., et al. (2019). IQGAP1 promotes pancreatic cancer progression and epithelial-mesenchymal transition (EMT) through Wnt/β-catenin signaling. Scientific Reports, 9 (1), 7539.
Siersbæk, R., et al. (2020). IL6/STAT3 signaling hijacks estrogen receptor α enhancers to drive breast cancer metastasis. Cancer Cell, 38 (3), 412–423.e9.
Gray, J. W. (2003). Evidence emerges for early metastasis and parallel evolution of primary and metastatic tumors. Cancer Cell, 4 (1), 4–6.
Fearon, E. R., & Vogelstein, B. (1990). A genetic model for colorectal tumorigenesis. Cell, 61 (5), 759–767.
Cairns, J. (1975). Mutation selection and the natural history of cancer. Nature, 255 (5505), 197–200.
Friberg, S., & Nyström, A. (2015). Cancer metastases: Early dissemination and late recurrences. Cancer Growth Metastasis, 8 , 43–49.
Collins, V. P., Loeffler, R. K., & Tivey, H. (1956). Observations on growth rates of human tumors. The American Journal of Roentgenology, Radium Therapy, and Nuclear Medicine, 76 (5), 988–1000.
Friberg, S., & Mattson, S. (1997). On the growth rates of human malignant tumors: Implications for medical decision making. Journal of Surgical Oncology, 65 (4), 284–297.
Zehir, A., et al. (2017). Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nature Medicine, 23 (6), 703–713.
Garcia-Recio, S., et al. (2023). Multiomics in primary and metastatic breast tumors from the AURORA US network finds microenvironment and epigenetic drivers of metastasis. Nature Cancer, 4 (1), 128–147.
Li, Z., et al. (2019). Metastatic breast cancers have reduced immune cell recruitment but harbor increased macrophages relative to their matched primary tumors. Journal for Immunotherapy of Cancer, 7 (1), 265.
Castaño, Z., et al. (2018). IL-1β inflammatory response driven by primary breast cancer prevents metastasis-initiating cell colonization. Nature Cell Biology, 20 (9), 1084–1097.
Martínez-Jiménez, F., et al. (2023). Genetic immune escape landscape in primary and metastatic cancer. Nature Genetics, 55 (5), 820–831.
Martínez-Jiménez, F., et al. (2023). Pan-cancer whole-genome comparison of primary and metastatic solid tumours. Nature, 618 (7964), 333–341.
Boman, C., et al. (2021). Discordance of PD-L1 status between primary and metastatic breast cancer: A systematic review and meta-analysis. Cancer Treatment Reviews, 99 , 102257.
Paget, S. (1989). The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Reviews, 8 (2), 98–101.
Langley, R. R., & Fidler, I. J. (2011). The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. International Journal of Cancer, 128 (11), 2527–2535.
Akhtar, M., et al. (2019). Paget’s “seed and soil” theory of cancer metastasis: An idea whose time has come. Advances in Anatomic Pathology, 26 (1).
Couto, J. P., et al. (2023). Nicotinamide N-methyltransferase sustains a core epigenetic program that promotes metastatic colonization in breast cancer. The EMBO Journal, 42 (13), e112559.
Poltavets, V., et al. (2018). The role of the extracellular matrix and its molecular and cellular regulators in cancer cell plasticity. Frontiers in Oncology, 8 , 431.
Cameron, M. D., et al. (2000). Temporal progression of metastasis in lung: Cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Research, 60 (9), 2541–2546.
Zomer, A., et al. (2015). In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell, 161 (5), 1046–1057.
Shiao, S. L., et al. (2011). Immune microenvironments in solid tumors: New targets for therapy. Genes & Development, 25 (24), 2559–2572.
Article CAS Google Scholar
Jahanban-Esfahlan, R., et al. (2019). Tumor cell dormancy: Threat or opportunity in the fight against cancer. Cancers (Basel), 11 (8).
Cox, T. R., Gartland, A., & Erler, J. T. (2012). The pre-metastatic niche: Is metastasis random? Bonekey Rep, 1 , 80.
Liu, Y., & Cao, X. (2016). Characteristics and significance of the pre-metastatic niche. Cancer Cell, 30 (5), 668–681.
Guo, Y., et al. (2019). Effects of exosomes on pre-metastatic niche formation in tumors. Molecular Cancer, 18 (1), 39.
Lobb, R. J., Lima, L. G., & Möller, A. (2017). Exosomes: Key mediators of metastasis and pre-metastatic niche formation. Seminars in Cell & Developmental Biology, 67 , 3–10.
Weidle, U. H., et al. (2017). The multiple roles of exosomes in metastasis. Cancer Genomics Proteomics, 14 (1), 1–15.
Soung, Y. H., et al. (2016). Emerging roles of exosomes in cancer invasion and metastasis. BMB Reports, 49 (1), 18–25.
Zhou, W., et al. (2014). Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell, 25 (4), 501–515.
Ono, M., et al. (2014). Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Science Signaling, 7 (332), ra63.
Chu, G. C., et al. (2014). RANK- and c-Met-mediated signal network promotes prostate cancer metastatic colonization. Endocrine-Related Cancer, 21 (2), 311–326.
Wu, J. B., et al. (2017). MAOA-dependent activation of Shh-IL6-RANKL signaling network promotes prostate cancer metastasis by engaging tumor-stromal cell interactions. Cancer Cell, 31 (3), 368–382.
Sethi, N., et al. (2011). Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell, 19 (2), 192–205.
Lee, J. W., et al. (2019). Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature, 567 (7747), 249–252.
Ji, Q., et al. (2020). Primary tumors release ITGBL1-rich extracellular vesicles to promote distal metastatic tumor growth through fibroblast-niche formation. Nature Communications, 11 (1), 1211.
Chow, A., et al. (2014). Macrophage immunomodulation by breast cancer-derived exosomes requires toll-like receptor 2-mediated activation of NF-κB. Scientific Reports, 4 , 5750.
Fang, T., et al. (2018). Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nature Communications, 9 (1), 191.
Ogawa, F., et al. (2014). Prostanoid induces premetastatic niche in regional lymph nodes. The Journal of Clinical Investigation, 124 (11), 4882–4894.
Kaplan, R. N., et al. (2005). VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature, 438 (7069), 820–827.
Wang, Z., et al. (2016). Periostin promotes immunosuppressive premetastatic niche formation to facilitate breast tumour metastasis. The Journal of Pathology, 239 (4), 484–495.
Wong, C. C., et al. (2011). Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proceedings of the National Academy of Sciences of the United States of America, 108 (39), 16369–16374.
Erler, J. T., et al. (2009). Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell, 15 (1), 35–44.
Cox, T. R., et al. (2015). The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature, 522 (7554), 106–110.
Rachman-Tzemah, C., et al. (2017). Blocking surgically induced lysyl oxidase activity reduces the risk of lung metastases. Cell Reports, 19 (4), 774–784.
Semenza, G. L. (2016). The hypoxic tumor microenvironment: A driving force for breast cancer progression. Biochimica et Biophysica Acta, 1863 (3), 382–391.
Gilkes, D. M., & Semenza, G. L. (2013). Role of hypoxia-inducible factors in breast cancer metastasis. Future Oncology, 9 (11), 1623–1636.
Todd, V. M., et al. (2021). Hypoxia inducible factor signaling in breast tumors controls spontaneous tumor dissemination in a site-specific manner. Communications Biology, 4 (1), 1122.
Yip, R. K. H., et al. (2021). Mammary tumour cells remodel the bone marrow vascular microenvironment to support metastasis. Nature Communications, 12 (1), 6920.
Huang, Y., et al. (2009). Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Research, 69 (19), 7529–7537.
Minami, T., et al. (2013). The calcineurin-NFAT-angiopoietin-2 signaling axis in lung endothelium is critical for the establishment of lung metastases. Cell Reports, 4 (4), 709–723.
Yan, H. H., et al. (2010). Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Research, 70 (15), 6139–6149.
Kai, F., Drain, A. P., & Weaver, V. M. (2019). The extracellular matrix modulates the metastatic journey. Developmental Cell, 49 (3), 332–346.
McFarlane, S., et al. (2015). CD44 increases the efficiency of distant metastasis of breast cancer. Oncotarget, 6 (13), 11465–11476.
Gerratana, L., et al. (2015). Pattern of metastasis and outcome in patients with breast cancer. Clinical & Experimental Metastasis, 32 (2), 125–133.
Wang, R., et al. (2019). The clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer, 19 (1), 1091.
Chen, W., et al. (2018). Organotropism: New insights into molecular mechanisms of breast cancer metastasis. NPJ Precis Oncol, 2 (1), 4.
Wu, Q., et al. (2017). Breast cancer subtypes predict the preferential site of distant metastases: A SEER based study. Oncotarget, 8 (17), 27990–27996.
Smith, H. A., & Kang, Y. (2017). Determinants of organotropic metastasis. Annual Review of Cancer Biology, 1 (1), 403–423.
Mo, Z., et al. (2021). Extracellular vesicle-associated organotropic metastasis. Cell Proliferation, 54 (1), e12948.
Hoshino, A., et al. (2015). Tumour exosome integrins determine organotropic metastasis. Nature, 527 (7578), 329–335.
Casimiro, S., Guise, T. A., & Chirgwin, J. (2009). The critical role of the bone microenvironment in cancer metastases. Molecular and Cellular Endocrinology, 310 (1-2), 71–81.
Guise, T. A. (2002). The vicious cycle of bone metastases. Journal of Musculoskeletal & Neuronal Interactions, 2 (6), 570–572.
CAS Google Scholar
Budczies, J., et al. (2015). The landscape of metastatic progression patterns across major human cancers. Oncotarget, 6 (1), 570–583.
Disibio, G., & French, S. W. (2008). Metastatic patterns of cancers: Results from a large autopsy study. Archives of Pathology & Laboratory Medicine, 132 (6), 931–939.
Rucci, N., & Teti, A. (2010). Osteomimicry: How tumor cells try to deceive the bone. Frontiers in Bioscience (Scholar Edition), 2 (3), 907–915.
Kennecke, H., et al. (2010). Metastatic behavior of breast cancer subtypes. Journal of Clinical Oncology, 28 (20), 3271–3277.
Bendinelli, P., et al. (2014). Microenvironmental stimuli affect endothelin-1 signaling responsible for invasiveness and osteomimicry of bone metastasis from breast cancer. Biochimica et Biophysica Acta, 1843 (4), 815–826.
Zhang, X. H., et al. (2009). Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell, 16 (1), 67–78.
Zhang, X. H., et al. (2013). Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell, 154 (5), 1060–1073.
Kumar, A., Sharma, P., & Sarin, S. K. (2008). Hepatic venous pressure gradient measurement: Time to learn! Indian Journal of Gastroenterology, 27 (2), 74–80.
MacPhee, P. J., Schmidt, E. E., & Groom, A. C. (1995). Intermittence of blood flow in liver sinusoids, studied by high-resolution in vivo microscopy. The American Journal of Physiology, 269 (5 Pt 1), G692–G698.
Kimbung, S., et al. (2016). Transcriptional profiling of breast cancer metastases identifies liver metastasis-selective genes associated with adverse outcome in luminal a primary breast cancer. Clinical Cancer Research, 22 (1), 146–157.
Minn, A. J., et al. (2005). Genes that mediate breast cancer metastasis to lung. Nature, 436 (7050), 518–524.
Bos, P. D., et al. (2009). Genes that mediate breast cancer metastasis to the brain. Nature, 459 (7249), 1005–1009.
Zhang, L., et al. (2011). MicroRNA-1258 suppresses breast cancer brain metastasis by targeting heparanase. Cancer Research, 71 (3), 645–654.
Valiente, M., et al. (2014). Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell, 156 (5), 1002–1016.
Gerratana, L., et al. (2021). Understanding the organ tropism of metastatic breast cancer through the combination of liquid biopsy tools. European Journal of Cancer, 143 , 147–157.
Marusyk, A., Almendro, V., & Polyak, K. (2012). Intra-tumour heterogeneity: A looking glass for cancer? Nature Reviews. Cancer, 12 (5), 323–334.
Su, C., et al. (2021). The key roles of cancer stem cell-derived extracellular vesicles. Signal Transduction and Targeted Therapy, 6 (1), 109.
Song, K., & Farzaneh, M. (2021). Signaling pathways governing breast cancer stem cells behavior. Stem Cell Research & Therapy, 12 (1), 245.
Kai, K., et al. (2010). Breast cancer stem cells. Breast Cancer, 17 (2), 80–85.
Charafe-Jauffret, E., et al. (2010). Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clinical Cancer Research, 16 (1), 45–55.
Grimshaw, M. J., et al. (2008). Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells. Breast Cancer Research, 10 (3), R52.
Al-Hajj, M., et al. (2003). Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America, 100 (7), 3983–3988.
Al-Hajj, M., & Clarke, M. F. (2004). Self-renewal and solid tumor stem cells. Oncogene, 23 (43), 7274–7282.
Butti, R., et al. (2019). Breast cancer stem cells: Biology and therapeutic implications. The International Journal of Biochemistry & Cell Biology, 107 , 38–52.
Badve, S., & Nakshatri, H. (2012). Breast-cancer stem cells-beyond semantics. The Lancet Oncology, 13 (1), e43–e48.
Liu, S., et al. (2014). Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Reports, 2 (1), 78–91.
Bao, L., et al. (2015). Multipotent luminal mammary cancer stem cells model tumor heterogeneity. Breast Cancer Research, 17 (1), 137.
Sin, W. C., & Lim, C. L. (2017). Breast cancer stem cells-from origins to targeted therapy. Stem Cell Investigation, 4 , 96.
da Silveira, W. A., et al. (2017). Transcription factor networks derived from breast cancer stem cells control the immune response in the basal subtype. Scientific Reports, 7 (1), 2851.
Vesuna, F., et al. (2009). Twist modulates breast cancer stem cells by transcriptional regulation of CD24 expression. Neoplasia, 11 (12), 1318–1328.
Ginestier, C., et al. (2007). ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell, 1 (5), 555–567.
Balamurugan, K., et al. (2019). C/EBPδ links IL-6 and HIF-1 signaling to promote breast cancer stem cell-associated phenotypes. Oncogene, 38 (20), 3765–3780.
da Silva-Diz, V., et al. (2018). Cancer cell plasticity: Impact on tumor progression and therapy response. Seminars in Cancer Biology, 53 , 48–58.
Jolly, M. K., et al. (2017). EMT and MET: Necessary or permissive for metastasis? Molecular Oncology, 11 (7), 755–769.
Yuan, S., Norgard, R. J., & Stanger, B. Z. (2019). Cellular plasticity in cancer. Cancer Discovery, 9 (7), 837–851.
Mani, S. A., et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell, 133 (4), 704–715.
Hermann, P. C., et al. (2007). Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell, 1 (3), 313–323.
Malanchi, I., et al. (2012). Interactions between cancer stem cells and their niche govern metastatic colonization. Nature, 481 (7379), 85–89.
May, C. D., et al. (2011). Epithelial-mesenchymal transition and cancer stem cells: A dangerously dynamic duo in breast cancer progression. Breast Cancer Research, 13 (1), 202.
Mallini, P., et al. (2014). Epithelial-to-mesenchymal transition: What is the impact on breast cancer stem cells and drug resistance. Cancer Treatment Reviews, 40 (3), 341–348.
Shuang, Z. Y., et al. (2014). Transforming growth factor-β1-induced epithelial-mesenchymal transition generates ALDH-positive cells with stem cell properties in cholangiocarcinoma. Cancer Letters, 354 (2), 320–328.
Wu, M., et al. (2022). Cancer stem cell regulated phenotypic plasticity protects metastasized cancer cells from ferroptosis. Nature Communications, 13 (1), 1371.
Bourcy, M., et al. (2016). Tissue factor induced by epithelial-mesenchymal transition triggers a procoagulant state that drives metastasis of circulating tumor cells. Cancer Research, 76 (14), 4270–4282.
Stankic, M., et al. (2013). TGF- β -Id1 signaling opposes Twist1 and promotes metastatic colonization via a mesenchymal-to-epithelial transition. Cell Reports, 5 (5), 1228–1242.
Ocaña, O. H., et al. (2012). Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell, 22 (6), 709–724.
Kröger, C., et al. (2019). Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America, 116 (15), 7353–7362.
Ford, H. L., & Thompson, E. W. (2010). Mammary gland studies as important contributors to the cause of epithelial mesenchymal plasticity in malignancy. Journal of Mammary Gland Biology and Neoplasia, 15 (2), 113–115.
Grosse-Wilde, A., et al. (2015). Stemness of the hybrid epithelial/mesenchymal state in breast cancer and its association with poor survival. PLoS One, 10 (5), e0126522.
Gandhi, N., & Das, G. M. (2019). Metabolic reprogramming in breast cancer and its therapeutic implications. Cells, 8 (2).
Sun, X., et al. (2020). Metabolic reprogramming in triple-negative breast cancer. Frontiers in Oncology, 10 , 428.
Lunetti, P., et al. (2019). Metabolic reprogramming in breast cancer results in distinct mitochondrial bioenergetics between luminal and basal subtypes. The FEBS Journal, 286 (4), 688–709.
Lehuédé, C., et al. (2016). Metabolic plasticity as a determinant of tumor growth and metastasis. Cancer Research, 76 (18), 5201–5208.
McGuirk, S., Audet-Delage, Y., & St-Pierre, J. (2020). Metabolic fitness and plasticity in cancer progression. Trends Cancer, 6 (1), 49–61.
Kamarajugadda, S., et al. (2013). Manganese superoxide dismutase promotes anoikis resistance and tumor metastasis. Cell Death & Disease, 4 (2), e504–e504.
Elia, I., et al. (2017). Proline metabolism supports metastasis formation and could be inhibited to selectively target metastasizing cancer cells. Nature Communications, 8 (1), 15267.
Andrzejewski, S., et al. (2017). PGC-1α promotes breast cancer metastasis and confers bioenergetic flexibility against metabolic drugs. Cell Metabolism, 26 (5), 778–787.
Weiss, L. (1990). Metastatic inefficiency. Advances in Cancer Research, 54 , 159–211.
Manjili, M. H. (2017). Tumor dormancy and relapse: From a natural byproduct of evolution to a disease state. Cancer Research, 77 (10), 2564–2569.
Werner, S., Heidrich, I., & Pantel, K. (2022). Clinical management and biology of tumor dormancy in breast cancer. Seminars in Cancer Biology, 78 , 49–62.
Pan, H., et al. (2017). 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. New England Journal of Medicine, 377 (19), 1836–1846.
Harper, K. L., et al. (2016). Mechanism of early dissemination and metastasis in HER2(+) mammary cancer. Nature, 540 (7634), 588–592.
McGranahan, N., & Swanton, C. (2017). Clonal heterogeneity and tumor evolution: Past, present, and the future. Cell, 168 (4), 613–628.
Celià-Terrassa, T., & Kang, Y. (2016). Distinctive properties of metastasis-initiating cells. Genes & Development, 30 (8), 892–908.
Ren, Q., et al. (2022). Gene expression predicts dormant metastatic breast cancer cell phenotype. Breast Cancer Research, 24 (1), 10.
Bidard, F. C., Proudhon, C., & Pierga, J. Y. (2016). Circulating tumor cells in breast cancer. Molecular Oncology, 10 (3), 418–430.
Bidard, F. C., et al. (2008). Disseminated tumor cells of breast cancer patients: A strong prognostic factor for distant and local relapse. Clinical Cancer Research, 14 (11), 3306–3311.
Janni, W., et al. (2011). Persistence of disseminated tumor cells in the bone marrow of breast cancer patients predicts increased risk for relapse--a European pooled analysis. Clinical Cancer Research, 17 (9), 2967–2976.
Hartkopf, A. D., et al. (2014). Prognostic relevance of disseminated tumour cells from the bone marrow of early stage breast cancer patients - results from a large single-centre analysis. European Journal of Cancer, 50 (15), 2550–2559.
Mathiesen, R. R., et al. (2012). Persistence of disseminated tumor cells after neoadjuvant treatment for locally advanced breast cancer predicts poor survival. Breast Cancer Research, 14 (4), R117.
Rueda, O. M., et al. (2019). Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups. Nature, 567 (7748), 399–404.
Gawrzak, S., et al. (2018). MSK1 regulates luminal cell differentiation and metastatic dormancy in ER+ breast cancer. Nature Cell Biology, 20 (2), 211–221.
Akkoc, Y., et al. (2021). Autophagy and cancer dormancy. Frontiers in Oncology, 11 , 627023.
Vera-Ramirez, L., et al. (2018). Autophagy promotes the survival of dormant breast cancer cells and metastatic tumour recurrence. Nature Communications, 9 (1), 1944.
Vogelstein, B., et al. (2013). Cancer genome landscapes. Science, 339 (6127), 1546–1558.
Casasent, A. K., et al. (2018). Multiclonal invasion in breast tumors identified by topographic single cell sequencing. Cell, 172 (1-2), 205–217.e12.
Makohon-Moore, A. P., et al. (2017). Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nature Genetics, 49 (3), 358–366.
Vanharanta, S., & Massagué, J. (2013). Origins of metastatic traits. Cancer Cell, 24 (4), 410–421.
Patel, S. A., & Vanharanta, S. (2017). Epigenetic determinants of metastasis. Molecular Oncology, 11 (1), 79–96.
Chatterjee, A., Rodger, E. J., & Eccles, M. R. (2018). Epigenetic drivers of tumourigenesis and cancer metastasis. Seminars in Cancer Biology, 51 , 149–159.
Cao, J., & Yan, Q. (2013). Histone demethylases set the stage for cancer metastasis. Science Signaling, 6 (273), pe15.
Cock-Rada, A., & Weitzman, J. B. (2013). The methylation landscape of tumour metastasis. Biology of the Cell, 105 (2), 73–90.
Flavahan, W. A., Gaskell, E., & Bernstein, B. E. (2017). Epigenetic plasticity and the hallmarks of cancer. Science, 357 (6348).
Easwaran, H., Tsai, H. C., & Baylin, S. B. (2014). Cancer epigenetics: Tumor heterogeneity, plasticity of stem-like states, and drug resistance. Molecular Cell, 54 (5), 716–727.
Saleh, R., et al. (2020). Role of epigenetic modifications in inhibitory immune checkpoints in cancer development and progression. Frontiers in Immunology, 11 , 1469.
Thakur, C., et al. (2022). Epigenetics and environment in breast cancer: New paradigms for anti-cancer therapies. Frontiers in Oncology, 12 , 971288.
Nass, S. J., et al. (2000). Aberrant methylation of the estrogen receptor and E-cadherin 5' CpG islands increases with malignant progression in human breast cancer. Cancer Research, 60 (16), 4346–4348.
Yang, J., & Weinberg, R. A. (2008). Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Developmental Cell, 14 (6), 818–829.
Prasad, C. P., et al. (2008). Epigenetic alterations of CDH1 and APC genes: Relationship with activation of Wnt/beta-catenin pathway in invasive ductal carcinoma of breast. Life Sciences, 83 (9-10), 318–325.
Serrano-Gomez, S. J., Maziveyi, M., & Alahari, S. K. (2016). Regulation of epithelial-mesenchymal transition through epigenetic and post-translational modifications. Molecular Cancer, 15 , 18.
Maroni, P., et al. (2017). Functions and epigenetic regulation of Wwox in bone metastasis from breast carcinoma: Comparison with primary tumors. International Journal of Molecular Sciences, 18 (1), 75.
Tiwari, N., et al. (2013). Sox4 is a master regulator of epithelial-mesenchymal transition by controlling Ezh2 expression and epigenetic reprogramming. Cancer Cell, 23 (6), 768–783.
Yu, W., et al. (2019). GATA3 recruits UTX for gene transcriptional activation to suppress metastasis of breast cancer. Cell Death & Disease, 10 (11), 832.
Imani, S., et al. (2016). Prognostic value of EMT-inducing transcription factors (EMT-TFs) in metastatic breast cancer: A systematic review and meta-analysis. Scientific Reports, 6 , 28587.
Kallergi, G., et al. (2011). Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Research, 13 (3), R59.
Dong, C., et al. (2013). Interaction with Suv39H1 is critical for Snail-mediated E-cadherin repression in breast cancer. Oncogene, 32 (11), 1351–1362.
Cai, W. L., et al. (2020). Specific chromatin landscapes and transcription factors couple breast cancer subtype with metastatic relapse to lung or brain. BMC Medical Genomics, 13 (1), 33.
Zucchetti, B., et al. (2019). The role of histone deacetylase inhibitors in metastatic breast cancer. Breast, 43 , 130–134.
Moore, L. D., Le, T., & Fan, G. (2013). DNA methylation and its basic function. Neuropsychopharmacology, 38 (1), 23–38.
Martin, E. M., & Fry, R. C. (2018). Environmental influences on the epigenome: Exposure- associated DNA methylation in human populations. Annual Review of Public Health, 39 (1), 309–333.
Bombonati, A., & Sgroi, D. C. (2011). The molecular pathology of breast cancer progression. The Journal of Pathology, 223 (2), 307–317.
Baylin, S. B., et al. (2001). Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Human Molecular Genetics, 10 (7), 687–692.
Narayan, A., et al. (1998). Hypomethylation of pericentromeric DNA in breast adenocarcinomas. International Journal of Cancer, 77 (6), 833–838.
Zhu, X., et al. (2015). Hypermethylation of BRCA1 gene: Implication for prognostic biomarker and therapeutic target in sporadic primary triple-negative breast cancer. Breast Cancer Research and Treatment, 150 , 479–486.
Shargh, S. A., et al. (2014). Downregulation of E-cadherin expression in breast cancer by promoter hypermethylation and its relation with progression and prognosis of tumor. Medical Oncology, 31 , 1–6.
Mirza, S., et al. (2007). Promoter hypermethylation of TMS1, BRCA1, ERα and PRB in serum and tumor DNA of invasive ductal breast carcinoma patients. Life Sciences, 81 (4), 280–287.
Locke, W. J., et al. (2015). Coordinated epigenetic remodelling of transcriptional networks occurs during early breast carcinogenesis. Clinical Epigenetics, 7 (1), 1–15.
Esteller, M., et al. (2000). Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. JNCI: Journal of the National Cancer Institute, 92 (7), 564–569.
Fujikane, T., et al. (2010). Genomic screening for genes upregulated by demethylation revealed novel targets of epigenetic silencing in breast cancer. Breast Cancer Research and Treatment, 122 , 699–710.
Radpour, R., et al. (2011). Integrated epigenetics of human breast cancer: Synoptic investigation of targeted genes, microRNAs and proteins upon demethylation treatment. PLoS One, 6 (11), e27355.
Butcher, D. T., & Rodenhiser, D. I. (2007). Epigenetic inactivation of BRCA1 is associated with aberrant expression of CTCF and DNA methyltransferase (DNMT3B) in some sporadic breast tumours. European Journal of Cancer, 43 (1), 210–219.
Vallian, S., et al. (2009). Methylation status of p16 INK4A tumor suppressor gene in Iranian patients with sporadic breast cancer. Journal of Cancer Research and Clinical Oncology, 135 , 991–996.
de Almeida, B. P., et al. (2019). Roadmap of DNA methylation in breast cancer identifies novel prognostic biomarkers. BMC Cancer, 19 (1), 219.
Batra, R. N., et al. (2021). DNA methylation landscapes of 1538 breast cancers reveal a replication-linked clock, epigenomic instability and cis-regulation. Nature Communications, 12 (1), 5406.
Wang, S.-C. M., Dowhan, D. H., & Muscat, G. E. (2019). Epigenetic arginine methylation in breast cancer: Emerging therapeutic strategies. Journal of Molecular Endocrinology, 62 (3), R223–R237.
Wu, Y. S., et al. (2019). Epigenetics in metastatic breast cancer: Its regulation and implications in diagnosis, prognosis and therapeutics. Current Cancer Drug Targets, 19 (2), 82–100.
Huang, T. H.-M., et al. (1997). Identification of DNA methylation markers for human breast carcinomas using the methylation-sensitive restriction fingerprinting technique. Cancer Research, 57 (6), 1030–1034.
Chen, C.-M., et al. (2003). Methylation target array for rapid analysis of CpG island hypermethylation in multiple tissue genomes. The American Journal of Pathology, 163 (1), 37–45.
Huang, T. H.-M., Perry, M. R., & Laux, D. E. (1999). Methylation profiling of CpG islands in human breast cancer cells. Human Molecular Genetics, 8 (3), 459–470.
Fackler, M. J., et al. (2020). DNA methylation markers predict recurrence-free interval in triple-negative breast cancer. NPJ Breast Cancer, 6 (1), 3.
Mendaza, S., et al. (2020). ADAM12 is a potential therapeutic target regulated by hypomethylation in triple-negative breast cancer. International Journal of Molecular Sciences, 21 (3), 903.
Li, W., et al. (2019). Epigenetic hypomethylation and upregulation of GD3s in triple negative breast cancer. Annals of Translational Medicine, 7 (23).
Hu, X.-C., Wong, I. H., & Chow, L. W. (2003). Tumor-derived aberrant methylation in plasma of invasive ductal breast cancer patients: Clinical implications. Oncology Reports, 10 (6), 1811–1815.
Graff, J. R., et al. (2000). Methylation patterns of the E-cadherin 5′ CpG island are unstable and reflect the dynamic, heterogeneous loss of E-cadherin expression during metastatic progression. Journal of Biological Chemistry, 275 (4), 2727–2732.
Bachman, K. E., et al. (1999). Methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene suggests a suppressor role in kidney, brain, and other human cancers. Cancer Research, 59 (4), 798–802.
Hansel, D. E., et al. (2003). MAGE1 is expressed by a subset of pancreatic endocrine neoplasms and associated lymph node and liver metastases. International Journal of Gastrointestinal Cancer, 33 , 141–147.
Kavalar, R., et al. (2001). Expression of MAGE tumour-associated antigens is inversely correlated with tumour differentiation in invasive ductal breast cancers: An immunohistochemical study. Virchows Archiv, 439 , 127–131.
Brasseur, F., et al. (1992). Human gene MAGE-1, which codes for a tumor-rejection antigen, is expressed by some breast tumors. International Journal of Cancer, 52 (5), 839–841.
Taback, B., et al. (2001). Detection of occult metastatic breast cancer cells in blood by a multimolecular marker assay: Correlation with clinical stage of disease. Cancer Research, 61 (24), 8845–8850.
Thakur, C., et al. (2022). Deletion of mdig enhances H3K36me3 and metastatic potential of the triple negative breast cancer cells. iScience, 25 (10), 105057.
Fisher, J., et al. (2000). Urokinase plasminogen activator system gene expression is increased in human breast carcinoma and its bone metastases—a comparison of normal breast tissue, non-invasive and invasive carcinoma and osseous metastases. Breast Cancer Research and Treatment, 61 , 1–12.
Guo, Y., et al. (2002). Regulation of DNA methylation in human breast cancer: Effect on the urokinase-type plasminogen activator gene production and tumor invasion. Journal of Biological Chemistry, 277 (44), 41571–41579.
Lu, A., et al. (2001). Molecular mechanisms for aberrant expression of the human breast cancer specific gene 1 in breast cancer cells: Control of transcription by DNA methylation and intronic sequences. Oncogene, 20 (37), 5173–5185.
Gupta, A., et al. (2003). Hypomethylation of the synuclein γ gene CpG island promotes its aberrant expression in breast carcinoma and ovarian carcinoma. Cancer Research, 63 (3), 664–673.
Albertazzi, E., et al. (1998). Expression of metastasis-associated genes h-mts1 (S100A4) and nm23 in carcinoma of breast is related to disease progression. DNA and Cell Biology, 17 (4), 335–342.
Yang, H., et al. (2019). A four-gene signature for prognosis in breast cancer patients with hypermethylated IL15RA. Oncology Letters, 17 (5), 4245–4254.
CAS PubMed PubMed Central Google Scholar
Stewart, C. M., & Tsui, D. W. (2018). Circulating cell-free DNA for non-invasive cancer management. Cancer Genetics, 228 , 169–179.
Xu, Z., Sandler, D. P., & Taylor, J. A. (2020). Blood DNA methylation and breast cancer: A prospective case-cohort analysis in the sister study. Journal of the National Cancer Institute, 112 (1), 87–94.
Manoochehri, M., et al. (2023). DNA methylation biomarkers for noninvasive detection of triple-negative breast cancer using liquid biopsy. International Journal of Cancer, 152 (5), 1025–1035.
Chimonidou, M., et al. (2017). Direct comparison study of DNA methylation markers in EpCAM-positive circulating tumour cells, corresponding circulating tumour DNA, and paired primary tumours in breast cancer. Oncotarget, 8 (42), 72054.
Bao-Caamano, A., Rodriguez-Casanova, A., & Diaz-Lagares, A. (2020). Epigenetics of circulating tumor cells in breast cancer. Advances in Experimental Medicine and Biology, 1220 , 117–134.
Singh, R., et al. (2018). Replication-dependent histone isoforms: A new source of complexity in chromatin structure and function. Nucleic Acids Research, 46 (17), 8665–8678.
Ahuja, N., Sharma, A. R., & Baylin, S. B. (2016). Epigenetic therapeutics: A new weapon in the war against cancer. Annual Review of Medicine, 67 , 73–89.
Blair, L. P., & Yan, Q. (2012). Epigenetic mechanisms in commonly occurring cancers. DNA and Cell Biology, 31 , S49–S61.
Healey, M. A., et al. (2014). Association of H3K9me3 and H3K27me3 repressive histone marks with breast cancer subtypes in the Nurses' Health Study. Breast Cancer Research and Treatment, 147 (3), 639–651.
Judes, G., et al. (2016). H3K4 acetylation, H3K9 acetylation and H3K27 methylation in breast tumor molecular subtypes. Epigenomics, 8 (7), 909–924.
Elsheikh, S. E., et al. (2009). Global histone modifications in breast cancer correlate with tumor phenotypes, prognostic factors, and patient outcome. Cancer Research, 69 (9), 3802–3809.
Spangle, J. M., et al. (2016). PI3K/AKT signaling regulates H3K4 methylation in breast cancer. Cell Reports, 15 (12), 2692–2704.
Mese, G., & Yalcin-Ozuysal, O. (2016). Epigenetics of breast cancer: DNA methylome and global histone modifications. Epigenetic Advancements in Cancer , 207–228.
Wei, Y., et al. (2008). Loss of trimethylation at lysine 27 of histone H3 is a predictor of poor outcome in breast, ovarian, and pancreatic cancers. Molecular Carcinogenesis, 47 (9), 701–706.
Yoo, K. H., & Hennighausen, L. (2012). EZH2 methyltransferase and H3K27 methylation in breast cancer. International Journal of Biological Sciences, 8 (1), 59–65.
Nassa, G., et al. (2019). Inhibition of histone methyltransferase DOT1L silences ERα gene and blocks proliferation of antiestrogen-resistant breast cancer cells. Science Advances, 5 (2), eaav5590.
Cho, M. H., et al. (2015). DOT1L cooperates with the c-Myc-p300 complex to epigenetically derepress CDH1 transcription factors in breast cancer progression. Nature Communications, 6 , 7821.
Wang, S. M., Dowhan, D. H., & Muscat, G. E. O. (2019). Epigenetic arginine methylation in breast cancer: Emerging therapeutic strategies. Journal of Molecular Endocrinology, 62 (3), R223–r237.
Yi, X., et al. (2017). EZH2-mediated epigenetic silencing of TIMP2 promotes ovarian cancer migration and invasion. Scientific Reports, 7 (1), 3568.
Mahmoud, F., et al. (2016). Role of EZH2 histone methyltrasferase in melanoma progression and metastasis. Cancer Biology & Therapy, 17 (6), 579–591.
Zhao, Y., et al. (2019). EZH2 cooperates with gain-of-function p53 mutants to promote cancer growth and metastasis. The EMBO Journal, 38 (5).
Gan, L., et al. (2018). Epigenetic regulation of cancer progression by EZH2: From biological insights to therapeutic potential. Biomarker Research, 6 (1), 1–10.
Holm, K., et al. (2012). Global H3K27 trimethylation and EZH2 abundance in breast tumor subtypes. Molecular Oncology, 6 (5), 494–506.
Zhang, W., et al. (2021). The bone microenvironment invigorates metastatic seeds for further dissemination. Cell, 184 (9), 2471–2486.e20.
Li, Z., et al. (2020). Methylation of EZH2 by PRMT1 regulates its stability and promotes breast cancer metastasis. Cell Death and Differentiation, 27 (12), 3226–3242.
Wang, Y., et al. (2009). LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell, 138 (4), 660–672.
Li, Q., et al. (2011). Binding of the JmjC demethylase JARID1B to LSD1/NuRD suppresses angiogenesis and metastasis in breast cancer cells by repressing chemokine CCL14. Cancer Research, 71 (21), 6899–6908.
Cao, J., et al. (2014). Histone demethylase RBP2 is critical for breast cancer progression and metastasis. Cell Reports, 6 (5), 868–877.
Luo, W., et al. (2012). Histone demethylase JMJD2C is a coactivator for hypoxia-inducible factor 1 that is required for breast cancer progression. Proceedings of the National Academy of Sciences of the United States of America, 109 (49), E3367–E3376.
Liu, R., et al. (2018). PHD finger protein 1 (PHF1) is a novel reader for histone H4R3 symmetric dimethylation and coordinates with PRMT5-WDR77/CRL4B complex to promote tumorigenesis. Nucleic Acids Research, 46 (13), 6608–6626.
Yao, R., et al. (2014). PRMT7 induces epithelial-to-mesenchymal transition and promotes metastasis in breast cancer. Cancer Research, 74 (19), 5656–5667.
Guo, P., et al. (2018). The histone acetylation modifications of breast cancer and their therapeutic implications. Pathology Oncology Research, 24 (4), 807–813.
Hałasa, M., et al. (2019). H3K18Ac as a marker of cancer progression and potential target of anti-cancer therapy. Cells, 8 (5).
Dong, H., et al. (2019). Activation of LncRNA TINCR by H3K27 acetylation promotes trastuzumab resistance and epithelial-mesenchymal transition by targeting microRNA-125b in breast cancer. Molecular Cancer, 18 (1), 3.
Bellucci, L., et al. (2013). Activation of p21 by HDAC inhibitors requires acetylation of H2A.Z. PLoS One, 8 (1), e54102.
Wang, L., et al. (2014). CARM1 methylates chromatin remodeling factor BAF155 to enhance tumor progression and metastasis. Cancer Cell, 25 (1), 21–36.
Nandy, D., Rajam, S. M., & Dutta, D. (2020). A three layered histone epigenetics in breast cancer metastasis. Cell & Bioscience, 10 (1), 52.
Gupta, R. A., et al. (2010). Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature, 464 (7291), 1071–1076.
Jiang, R., et al. (2017). The long noncoding RNA lnc-EGFR stimulates T-regulatory cells differentiation thus promoting hepatocellular carcinoma immune evasion. Nature Communications, 8 , 15129.
Chen, C., et al. (2018). LNMAT1 promotes lymphatic metastasis of bladder cancer via CCL2 dependent macrophage recruitment. Nature Communications, 9 (1), 3826.
Huang, D., et al. (2018). NKILA lncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death. Nature Immunology, 19 (10), 1112–1125.
Sang, L. J., et al. (2018). LncRNA CamK-A regulates Ca(2+)-signaling-mediated tumor microenvironment remodeling. Molecular Cell, 72 (3), 601.
Pencheva, N., & Tavazoie, S. F. (2013). Control of metastatic progression by microRNA regulatory networks. Nature Cell Biology, 15 (6), 546–554.
Chang, G., et al. (2020). YTHDF3 induces the translation of m(6)A-enriched gene transcripts to promote breast cancer brain metastasis. Cancer Cell, 38 (6), 857–871.e7.
Cheng, Y., et al. (2019). Targeting epigenetic regulators for cancer therapy: Mechanisms and advances in clinical trials. Signal Transduction and Targeted Therapy, 4 , 62.
Zhao, L., Pang, A., & Li, Y. (2018). Function of GCN5 in the TGF-β1-induced epithelial-to-mesenchymal transition in breast cancer. Oncology Letters, 16 (3), 3955–3963.
PubMed PubMed Central Google Scholar
Wang, L. T., et al. (2020). PCAF-mediated acetylation of ISX recruits BRD4 to promote epithelial-mesenchymal transition. EMBO Reports, 21 (2), e48795.
Tang, Z., et al. (2017). HDAC1 triggers the proliferation and migration of breast cancer cells via upregulation of interleukin-8. Biological Chemistry, 398 (12), 1347–1356.
Kamarulzaman, N. S., et al. (2017). The role of REST and HDAC2 in epigenetic dysregulation of Nav1.5 and nNav1.5 expression in breast cancer. Cancer Cell International, 17 , 74.
Kwak, S. M., et al. (2019). EGFR-c-Src-mediated HDAC3 phosphorylation exacerbates invasion of breast cancer cells. Cells, 8 (8).
Tang, X., et al. (2020). HDAC8 cooperates with SMAD3/4 complex to suppress SIRT7 and promote cell survival and migration. Nucleic Acids Research, 48 (6), 2912–2923.
Guerriero, J. L., et al. (2017). Class IIa HDAC inhibition reduces breast tumours and metastases through anti-tumour macrophages. Nature, 543 (7645), 428–432.
Ferrer, C. M., et al. (2017). O-GlcNAcylation regulates breast cancer metastasis via SIRT1 modulation of FOXM1 pathway. Oncogene, 36 (4), 559–569.
Lee, J. J., et al. (2018). Inhibition of epithelial cell migration and Src/FAK signaling by SIRT3. Proceedings of the National Academy of Sciences of the United States of America, 115 (27), 7057–7062.
Tang, X., et al. (2017). SIRT7 antagonizes TGF-β signaling and inhibits breast cancer metastasis. Nature Communications, 8 (1), 318.
Li, Z., et al. (2017). The degradation of EZH2 mediated by lncRNA ANCR attenuated the invasion and metastasis of breast cancer. Cell Death and Differentiation, 24 (1), 59–71.
Chiang, K., et al. (2017). PRMT5 is a critical regulator of breast cancer stem cell function via histone methylation and FOXP1 expression. Cell Reports, 21 (12), 3498–3513.
Chiang, K., & Davies, C. C. (2018). Linking PRMT5 to breast cancer stem cells: New therapeutic opportunities? Molecular & Cellular Oncology, 5 (3), e1441628.
Nakakido, M., et al. (2015). PRMT6 increases cytoplasmic localization of p21CDKN1A in cancer cells through arginine methylation and makes more resistant to cytotoxic agents. Oncotarget, 6 (31), 30957–30967.
Liu, Y., et al. (2020). Arginine methylation of SHANK2 by PRMT7 promotes human breast cancer metastasis through activating endosomal FAK signalling. eLife, 9 , e57617.
Hon, G. C., et al. (2012). Global DNA hypomethylation coupled to repressive chromatin domain formation and gene silencing in breast cancer. Genome Research, 22 (2), 246–258.
Thakur, C., et al. (2018). Loss of mdig expression enhances DNA and histone methylation and metastasis of aggressive breast cancer. Signal Transduction and Targeted Therapy, 3 , 25.
Majumder, A., et al. (2018). Enhanced expression of histone chaperone APLF associate with breast cancer. Molecular Cancer, 17 (1), 76.
Xiong, Z., et al. (2018). ANP32E induces tumorigenesis of triple-negative breast cancer cells by upregulating E2F1. Molecular Oncology, 12 (6), 896–912.
Hu, H., et al. (2011). Structure of a CENP-A-histone H4 heterodimer in complex with chaperone HJURP. Genes & Development, 25 (9), 901–906.
Montes de Oca, R., et al. (2015). The histone chaperone HJURP is a new independent prognostic marker for luminal A breast carcinoma. Molecular Oncology, 9 (3), 657–674.
Hu, Z., et al. (2010). The expression level of HJURP has an independent prognostic impact and predicts the sensitivity to radiotherapy in breast cancer. Breast Cancer Research, 12 (2), R18.
Coates, P., Dewar, J., & Thompson, A. M. (2010). At last, a predictive and prognostic marker for radiotherapy? Breast Cancer Research, 12 (3), 106.
Corpet, A., et al. (2011). Asf1b, the necessary Asf1 isoform for proliferation, is predictive of outcome in breast cancer. The EMBO Journal, 30 (3), 480–493.
Zeng, D., et al. (2019). Knockdown of nucleophosmin 1 suppresses proliferation of triple-negative breast cancer cells through activating CDH1/Skp2/p27kip1 pathway. Cancer Management and Research, 11 , 143–156.
Privette Vinnedge, L. M., et al. (2015). The DEK oncogene promotes cellular proliferation through paracrine Wnt signaling in Ron receptor-positive breast cancers. Oncogene, 34 (18), 2325–2336.
Shi, Y., et al. (2019). DAXX, as a tumor suppressor, impacts DNA damage repair and sensitizes BRCA-proficient TNBC cells to PARP inhibitors. Neoplasia, 21 (6), 533–544.
Garcia-Martinez, L., et al. (2021). Epigenetic mechanisms in breast cancer therapy and resistance. Nature Communications, 12 (1), 1786.
Huang, L., & Pardee, A. B. (2000). Suberoylanilide hydroxamic acid as a potential therapeutic agent for human breast cancer treatment. Molecular Medicine, 6 (10), 849–866.
Robertson, F. M., et al. (2010). Suberoylanilide hydroxamic acid blocks self-renewal and homotypic aggregation of inflammatory breast cancer spheroids. Cancer, 116 (11 Suppl), 2760–2767.
Thomas, S., et al. (2011). Addition of a histone deacetylase inhibitor redirects tamoxifen-treated breast cancer cells into apoptosis, which is opposed by the induction of autophagy. Breast Cancer Research and Treatment, 130 (2), 437–447.
Munster, P. N., et al. (2011). A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. British Journal of Cancer, 104 (12), 1828–1835.
Sabnis, G. J., et al. (2013). HDAC inhibitor entinostat restores responsiveness of letrozole-resistant MCF-7Ca xenografts to aromatase inhibitors through modulation of Her-2. Molecular Cancer Therapeutics, 12 (12), 2804–2816.
Alao, J. P., et al. (2004). Histone deacetylase inhibitor trichostatin A represses estrogen receptor alpha-dependent transcription and promotes proteasomal degradation of cyclin D1 in human breast carcinoma cell lines. Clinical Cancer Research, 10 (23), 8094–8104.
Fortunati, N., et al. (2008). Valproic acid is a selective antiproliferative agent in estrogen-sensitive breast cancer cells. Cancer Letters, 259 (2), 156–164.
Mawatari, T., et al. (2015). Valproic acid inhibits proliferation of HER2-expressing breast cancer cells by inducing cell cycle arrest and apoptosis through Hsp70 acetylation. International Journal of Oncology, 47 (6), 2073–2081.
Tomita, Y., et al. (2016). The interplay of epigenetic therapy and immunity in locally recurrent or metastatic estrogen receptor-positive breast cancer: Correlative analysis of ENCORE 301, a randomized, placebo-controlled phase II trial of exemestane with or without entinostat. Oncoimmunology, 5 (11), e1219008.
Qin, G., et al. (2019). Panobinostat (LBH589) inhibits Wnt/β-catenin signaling pathway via upregulating APCL expression in breast cancer. Cellular Signalling, 59 , 62–75.
Laengle, J., et al. (2020). Histone deacetylase inhibitors valproic acid and vorinostat enhance trastuzumab-mediated antibody-dependent cell-mediated phagocytosis. Journal for Immunotherapy of Cancer, 8 (1).
Damaskos, C., et al. (2017). Histone deacetylase inhibitors: An attractive therapeutic strategy against breast cancer. Anticancer Research, 37 (1), 35–46.
Zucchetti, B., et al. (2019). The role of histone deacetylase inhibitors in metastatic breast cancer. The Breast, 43 , 130–134.
Hu, Z., et al. (2023). Histone deacetylase inhibitors promote breast cancer metastasis by elevating NEDD9 expression. Signal Transduction and Targeted Therapy, 8 (1), 11.
Xiong, H., et al. (2009). Effects of 5-Aza-CdR on the proliferation of human breast cancer cell line MCF-7 and on the expression of Apaf-1 gene. Journal of Huazhong University of Science and Technology. Medical Sciences, 29 (4), 498–502.
Khan, G. N., et al. (2017). Azacytidine-induced chemosensitivity to doxorubicin in human breast cancer MCF7 cells. Anticancer Research, 37 (5), 2355–2364.
Butler, C., et al. (2020). Hypomethylating agent azacitidine is effective in treating brain metastasis triple-negative breast cancer through regulation of DNA methylation of keratin 18 gene. Translational Oncology, 13 (6), 100775.
Dahn, M. L., et al. (2020). Decitabine response in breast cancer requires efficient drug processing and is not limited by multidrug resistance. Molecular Cancer Therapeutics, 19 (5), 1110–1122.
Yu, J., et al. (2018). DNA methyltransferase expression in triple-negative breast cancer predicts sensitivity to decitabine. The Journal of Clinical Investigation, 128 (6), 2376–2388.
Vernier, M., et al. (2020). Inhibition of DNMT1 and ERRα crosstalk suppresses breast cancer via derepression of IRF4. Oncogene, 39 (41), 6406–6420.
Wong, K. K. (2021). DNMT1: A key drug target in triple-negative breast cancer. Seminars in Cancer Biology, 72 , 198–213.
Su, Y., et al. (2018). Epigenetic reprogramming of epithelial mesenchymal transition in triple negative breast cancer cells with DNA methyltransferase and histone deacetylase inhibitors. Journal of Experimental & Clinical Cancer Research, 37 (1), 314.
Muvarak, N. E., et al. (2016). Enhancing the cytotoxic effects of PARP inhibitors with DNA demethylating agents – a potential therapy for cancer. Cancer Cell, 30 (4), 637–650.
Terranova-Barberio, M., et al. (2017). HDAC inhibition potentiates immunotherapy in triple negative breast cancer. Oncotarget, 8 (69), 114156–114172.
Chequin, A., et al. (2021). Antitumoral activity of liraglutide, a new DNMT inhibitor in breast cancer cells in vitro and in vivo. Chemico-Biological Interactions, 349 , 109641.
Brown, L. J., et al. (2022). Epigenetic therapies and biomarkers in breast cancer. Cancers (Basel), 14 (3).
Thakur, C., & Chen, F. (2015). Current understanding of mdig/MINA in human cancers. Genes & Cancer, 6 (7-8), 288.
Zhang, Q., et al. (2019). New discoveries of mdig in the epigenetic regulation of cancers. In Seminars in cancer biology . Elsevier.
Google Scholar
Wu, K., et al. (2016). Proteomic characterization of the World Trade Center dust-activated mdig and c-myc signaling circuit linked to multiple myeloma. Scientific Reports, 6 (1), 36305.
Zhang, Y., et al. (2005). The human mineral dust-induced gene, mdig, is a cell growth regulating gene associated with lung cancer. Oncogene, 24 (31), 4873–4882.
Sun, J., et al. (2014). Carcinogenic metalloid arsenic induces expression of mdig oncogene through JNK and STAT3 activation. Cancer Letters, 346 (2), 257–263.
Thakur, C., et al. (2015). Oncoprotein mdig contributes to silica-induced pulmonary fibrosis by altering balance between Th17 and Treg T cells. Oncotarget, 6 (6), 3722.
Tsukada, Y. I., et al. (2006). Histone demethylation by a family of JmjC domain-containing proteins. Nature, 439 (7078), 811–816.
Lu, Y., et al. (2009). Lung cancer-associated JmjC domain protein mdig suppresses formation of tri-methyl lysine 9 of histone H3. Cell Cycle, 8 (13), 2101–2109.
Chen, B., et al. (2013). mdig de-represses H19 large intergenic non-coding RNA (lincRNA) by down-regulating H3K9me3 and heterochromatin. Oncotarget, 4 (9), 1427–1437.
Zhang, Q., et al. (2020). mdig promotes oncogenic gene expression through antagonizing repressive histone methylation markers. Theranostics, 10 (2), 602.
Thakur, C., et al. (2014). Increased expression of mdig predicts poorer survival of the breast cancer patients. Gene, 535 (2), 218–224.
Zhang, Q., et al. (2019). New discoveries of mdig in the epigenetic regulation of cancers. Seminars in Cancer Biology, 57 , 27–35.
Thakur, C., et al. (2018). Loss of mdig expression enhances DNA and histone methylation and metastasis of aggressive breast cancer. Signal Transduction and Targeted Therapy, 3 (1), 25.
Waks, A. G., & Winer, E. P. (2019). Breast cancer treatment: A review. Jama, 321 (3), 288–300.
Gkountela, S., et al. (2019). Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell, 176 (1-2), 98–112.e14.
Wu, A., et al. (2020). Genome-wide plasma DNA methylation features of metastatic prostate cancer. The Journal of Clinical Investigation, 130 (4), 1991–2000.
Download references
The research projects in FC’s laboratory are supported by the National Institutes of Health (NIH) grants R01 ES031822, R01 ES028335, and R01 ES028263 and the Research Start-up fund of Stony Brook University.
Author information
Authors and affiliations.
Stony Brook Cancer Center, Renaissance School of Medicine, Stony Brook University, Lauterbur Drive, Stony Brook, NY, 11794, USA
Chitra Thakur, Yiran Qiu, Aashna Pawar & Fei Chen
You can also search for this author in PubMed Google Scholar
Contributions
C.T. wrote the manuscript and designed the article.
YQ, AP, conducted the literature survey.
FC supervised the theme of the article and did the final proofreading.
Corresponding authors
Correspondence to Chitra Thakur or Fei Chen .
Ethics declarations
Ethical approval, informed consent, conflict of interest.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Thakur, C., Qiu, Y., Pawar, A. et al. Epigenetic regulation of breast cancer metastasis. Cancer Metastasis Rev 43 , 597–619 (2024). https://doi.org/10.1007/s10555-023-10146-7
Download citation
Received : 22 July 2023
Accepted : 02 October 2023
Published : 19 October 2023
Issue Date : June 2024
DOI : https://doi.org/10.1007/s10555-023-10146-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Epigenetic regulation
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Abstract. Breast cancer is the most common malignancy in women worldwide. Metastasis is the leading cause of high mortality in most cancers. Although predicting the early stage of breast cancer before metastasis can increase the survival rate, breast cancer is often discovered or diagnosed after metastasis has occurred.
Background: Differential efficacy of newly registered therapies in subgroups of metastatic breast cancer (mBC) is an important consideration for their subsequent use in clinical practice. In a systematic literature review, we evaluated differences in outcome regarding progression free survival (PFS), time to progression (TTP), overall survival (OS) and visceral versus non-visceral disease. The ...
The article presents a review of the literature on breast carcinoma - a disease affecting women in the world. Abstract. Breast cancer is the most-commonly diagnosed malignant tumor in women in the world, as well as the first cause of death from malignant tumors. The incidence of breast cancer is constantly increasing in all regions of the world.
Metastatic breast cancer is difficult to cure and has a worse prognosis with higher rates of mortality. ... in the case of painful metastasis, a large body of literature supports the use of the short course, ... Chua TC, Saxena A, Liauw W, Chu F, Morris DL. Hepatic resection for metastatic breast cancer: a systematic review. Eur J Cancer. 2011 ...
Metastatic breast cancer can be treated in specialized breast cancer centers, tumor centers and practices specialized in oncology. The teams that work there are made up of different kinds of health care specialists. ... The experience of living with metastatic breast cancer: a review of the literature. Health Care Women Int 2015; 36(5): 514-542 ...
Breast cancer is the most common malignancy in women worldwide. Metastasis is the leading cause of high mortality in most cancers. Although predicting the early stage of breast cancer before metastasis can increase the survival rate, breast cancer is often discovered or diagnosed after metastasis has occurred. In general, breast cancer has a poor prognosis because it starts as a local disease ...
However, the breast is rarely the site of metastatic disease. Trevithick was the first to report a case of extra-mammary breast metastasis in 1903. Metastasis in the breast can be misdiagnosed as a benign disease or primary malignancy and is usually a rare and unexpected diagnosis in a patient presenting with a breast mass.[5] The most commonly ...
Introduction. Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer-related death among women 1.Despite improved overall survival among patients with breast cancer 2, a significant number of patients eventually develop distant metastasis after initial treatment 3.The diagnosis of distant metastasis in patients with breast cancer is clinically and psychologically ...
This review discusses the mechanisms leading to metastasis and the current therapies to improve the early diagnosis and prognosis in patients with metastatic breast cancer. The FDA-approved drugs ...
Breast cancer is the most frequently diagnosed malignancy and the second leading cause of cancer-related mortality among women worldwide. Recurrent metastasis is associated with poor patient outcomes and poses a significant challenge in breast cancer therapies. Cancer cells adapting to a new tissue microenvironment is the key event in distant metastasis development, where the disseminating ...