An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


A clinician’s guide to conducting research on causal effects
Vivian h lyons , phd, mph, jamaica rm robinson , phd, mph, brianna mills , ma, phd, elizabeth y killien , md, mph, stephen j mooney , phd.
- Author information
- Article notes
- Copyright and License information
Author contributions : Drs. Lyons, Robinson, Mills, and Mooney conceptualized the project, provided background intellectual content, and drafted the initial manuscript. Dr. Killien provided clinical context and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Address correspondence to: Elizabeth Y. Killien, MD MPH, Seattle Children’s Hospital, Pediatric Critical Care Medicine FA 2.112, 4800 Sand Point Way NE, Seattle, WA 98105, [email protected] , (206) 987-5838.
Issue date 2022 Oct.
Surgeons are uniquely poised to conduct research to improve patient care, yet a gap often exists between the clinician’s desire to guide patient care with causal evidence and having adequate training necessary to produce causal evidence. This guide aims to address this gap by providing clinically relevant examples to illustrate necessary assumptions required for clinical research to produce causal estimates.
Keywords: Causality, Epidemiological Research Design, Clinical Research, Academic Surgery
Introduction
Surgeons working at the intersection of academics and clinical care are uniquely poised to conduct informed, relevant, and timely research to improve processes of care and patient outcomes. Research involvement can also broaden surgeons’ perspectives on their clinical work, satisfy intellectual curiosity, and aid in career development. 1 , 2 Additionally, research is often an explicit expectation of many clinical training programs and an essential component of academic promotion criteria.
While most medical students and academic physicians believe that participation in clinical research is important, 3 – 6 very few have formal training in research methodology 5 or sufficient knowledge of biostatistics to conduct rigorous research. 7 The number of clinicians participating in formal physician-scientist pathways or intensive Master’s or doctoral degree programs is declining. 8 Most clinicians first conduct research during non-degree-awarding academic residencies, fellowships, and other positions that may not include formal research training.
It is important to ensure that clinicians who have not undertaken additional advanced research training have the resources and support they need to conduct high quality clinical research. As epidemiologists working in an interdisciplinary injury research center, we often find ourselves working with surgeons who are caught between a desire to implement the best possible analytic strategy for a given research project and uncertainty around which strategy is the most appropriate. One of the most frequent challenges we have observed is confusion regarding causality in clinical research. This confusion includes understanding whether a research question is causal, when a statistical estimate may be interpreted as a causal effect , and how to best contribute to the evidence base when available data and statistical estimates aren’t suited to interpretation as causal effects.
Clinicians without formal research training may rely on simplifying rules-of-thumb regarding evidential value in clinical research. One such rule is that only randomized controlled trials produce causal evidence. 9 , 10 Yet, in practice, clinical decisions are informed by observations comprising the best available evidence rather than only evidence from randomized trials. 11 , 12 For example, one of the most common daily decisions surgeons must make is selection of appropriate analgesia for their patients while inpatient and upon discharge. While opioids have long been a mainstay of perioperative pain management, many hospitals have increased their scrutiny of opioid prescribing given concern about the worsening opioid epidemic. As a provider who must balance your patients’ analgesia requirements with these public health concerns, you would like to know whether opioid prescriptions after acute trauma or surgery are contributing to opioid misuse. You know that this isn’t a question that can be answered in a randomized controlled trial, but can you still assess it for a causal link? Our objective is for this guide to serve as an entry point to causal inference for a surgeon or other clinical practitioner with basic statistical knowledge and a research question about a potentially causal relationship.
What is a causal research question?
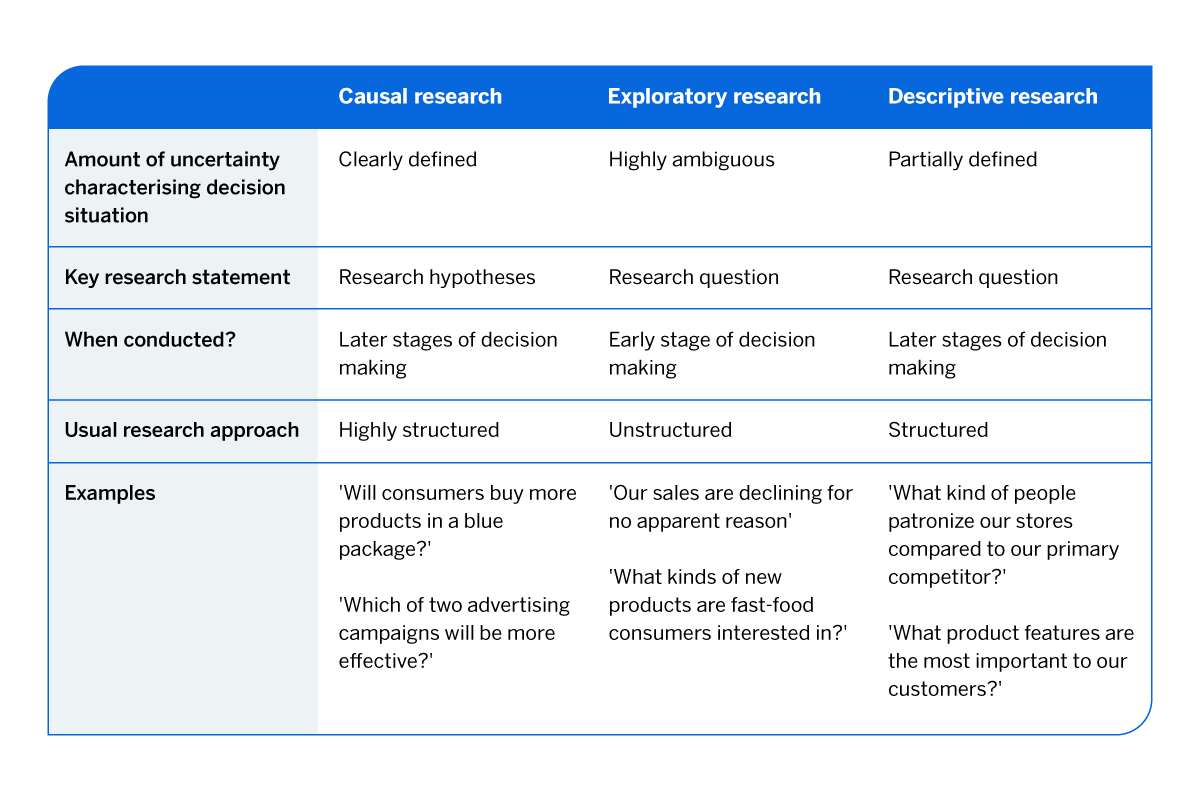
In general, epidemiologists roughly categorize clinical research questions as (1) descriptive, (2) predictive, or (3) causal. Understanding this taxonomy and how your research question fits into it can help you to select an appropriate analytic approach and linguistic framing for your project ( Table 1 ).
Categories of clinical research
Descriptive research characterizes distributions of disease prevalence, risk factors, or outcomes in a specific population, often within a specific time window. Findings from descriptive research provide a foundation for generating and refining hypotheses for future research endeavors, while also informing policymaking. 13 For example, you might design a study comparing the mean number of opioid prescriptions filled after hospitalizations for traumatic injury at different trauma facilities with rates of opioid abuse in that region. Descriptive studies may include comparisons (such as prevalence of opioid prescriptions at discharge by hospital type) but they do not support a counterfactual outcome (e.g., they do not ask how risk factor or disease distribution would differ if characteristics of the population or interventions were different).
Predictive research questions employ clinical data to predict outcomes for an individual patient or patient population given what is already known about that patient or population. For example, a clinical decision support algorithm developed from predictive research might forecast that a 65-year-old with a femur fracture with ongoing unresolved pain at discharge who is prescribed 30 days of opioids has a 20% one-year risk of developing long-term prescription opioid use. Unlike descriptive questions, which examine the present or the past, predictive research forecasts a specific future for an individual or defined population. However, like descriptive research, predictive research does not try to determine how the disease course or condition would change as the result of a different treatment choice.
Causal research questions ask how changes in health status result from changes in exposure or treatment. For example, a surgeon who asks if they should prescribe only non-steroidal anti-inflammatory drugs (NSAIDs) at discharge rather than opioids to discourage long-term opioid use is asking a causal question – if they change their behavior, will it cause a change in outcome? Or specifically, does an opioid prescription after traumatic injury contribute to risk of opioid use disorder? A key characteristic of this type of question is its counterfactual contrast. 14 Even though as a clinical researcher you observe, at most, one outcome for each individual (i.e., the patient developed long-term prescription opioid use or they did not develop long-term prescription opioid use), you are interested in projecting what the outcome would have been had the exposure been different than what it was; that is, if it were counter to fact (i.e., if the individual had taken non-opioid pain management versus the short course of prescribed opioids).
Ultimately, all three types of research are important and provide evidence for clinical decisions. However, the third research type, causal research, is the only type that demonstrates a direct effect of an intervention and is frequently the most challenging to conduct and interpret.
What is required for research to be causal?
Statistical analyses from any population-based study, including both observational studies and randomized trials, will typically estimate a controlled association between a treatment and an outcome. For example, to assess whether a history of prescribed opioids following an injury is associated with a higher risk of opioid overdose, your analysis might use statistics to hold every other measured patient characteristic (e.g., gender, age, baseline health status) constant, and identify that those with a history of prescribed opioids had five times the rate of opioid overdose when compared with those without a history of prescribed opioids.
Is this five-fold elevated risk the effect of prescribed opioids on opioid overdose incidence in the population that your study data comes from? Not necessarily. Even with a causal research question and a perfectly conducted research study of any design, a statistical parameter is not guaranteed to accurately estimate the population average causal effect. The plausibility that a statistical parameter (e.g. the five-fold risk observed above) represents a causal effect depends on a set of core assumptions. 15
Core assumptions
There are three core causal inference assumptions: (1) consistency, (2) positivity, and (3) conditional exchangeability.
Consistency is the assumption that your exposure, treatment, or intervention of interest is applied equally to all individuals classified as exposed, and not applied at all to individuals classified as unexposed. 16 If you wanted to compare outcomes among patients prescribed opioids to those prescribed NSAIDs at discharge, you might be concerned the consistency assumption would be violated due to variation in the specific opioid prescribed, the daily dose, the time interval between doses, the duration of the prescription, and how the prescribed dose changed over time.
Positivity is the assumption that there could be both exposed and unexposed people in each group of covariates on which you analytically stratify (e.g. age, gender, medical history), such that you are able to describe the distribution of the outcome across exposure levels in each covariate group. 17 For example, suppose your study was evaluating opioid use disorder incidence among patients initially prescribed opioids, adjusted for hospital and insurance status. If one of the included hospitals had a policy to prescribe lower cost NSAIDs rather than opioids to patients lacking health insurance, uninsured patients in the hospital that never prescribed opioids to uninsured patients would be systematically precluded from exposure status, violating the positivity assumption.
Conditional exchangeability is the assumption that, before treatment, exposed and unexposed individuals have equivalent probability of the outcome (conditional on covariates that have been controlled for). 14 In a study assessing whether opioid prescription use leads to increased risk of opioid dependency, it would help to have demographic information (e.g., age at prescription opioid initiation) and/or health status data (e.g., chronic pain) from participants. Satisfying conditional exchangeability requires an in-depth understanding of prior literature and theoretical frameworks that describe how relevant covariates influence the relationship between your exposure and outcome of interest.
If you have a causal research question, do you need to conduct a randomized controlled trial (RCT)?
In short: it’s nice if you can, but it’s not necessary. Clinicians and health researchers typically consider RCTs to be at the top of the ‘evidence pyramid,’ with good reason. Randomized controlled trials are designed to generate data where exposure (treatment) allocation meets the consistency assumption – by specifying the intervention that individuals receive, investigators hope to minimize differences in exposure to the point of being ignorable. Assigning the intervention usually also allows an RCT to meet the positivity assumption (every participate has a chance of being allocated the exposure) and, when randomization succeeds, the conditional exchangeability assumption (on average, the exposed group has the same predilection for the outcome as the unexposed group, except for the impact of the exposure). In short, RCTs are designed to increase the probability that those core assumptions will hold, allowing interpretation of statistical parameters as causal effects.
However, conducting an RCT may be unfeasible for an array of reasons, including lacking necessary financial or time resources, or having an exposure or hypothesis that is not possible or ethical to apply and/or alter for trial participants. It is also possible that the sample of willing participants may not be sufficiently representative of the broader patient population to produce meaningful results. 18 For example, you may consider enrolling patients into an RCT for an experimental opioid tapering protocol, but are concerned that patients prone to opioid use disorder would systematically decline to participate in the trial, which would result in estimating an effect that would not translate to the actual population of interest. Importantly, even RCTs are not guaranteed to meet core assumptions required for causal research. 19
If you have a causal research question and do not conduct an RCT, what makes a statistical parameter estimate interpretable as a causal effect?
If your study cannot meet the core assumptions of consistency, positivity, and conditional exchangeability – and observational studies usually cannot – your statistical estimates cannot be interpreted as causal effects. However, even if your estimates are not causal effects , they still can provide causal evidence . Evidence from controlled associations obtained with descriptive research provides foundational evidence for future causal hypotheses and research, and also may lead to clinical changes that can themselves be assessed more rigorously. 10 , 12 Consider that most of the evidence that smoking causes lung cancer is associational – as detailed previously, there are almost always issues with violation of all three core causal inference assumptions – but there are no plausible alternate causes for that relationship other than a causal effect of smoking.
Furthermore, consider that many of the methods used to approach a causal research question are neither necessary nor sufficient for answering the research question by themselves but do often provide valuable context for better understanding of the research question. 20 A Directed Acyclic Graph (DAG) is one such tool, which is used to graphically visualize the hypothesized causal relationships between the exposure, the outcome, and all related covariates. For example, say you want to estimate the impact of instituting a tapering protocol on opioid prescriptions and subsequent opioid dependency. Suppose you know that at your center, younger age is associated with being included in the tapering protocol, and that age may also affect the risk of developing an opioid addiction. Using this information to draw a DAG would not only illustrate the relationship hypothesized by the research question between the exposure and outcome of interest, but also the “back door path” through patient’s age that connects the opioid tapering protocol to opioid dependency ( Figure 1 ). 21 Since the focus of a DAG is on covariates that influence both the exposure and outcome, visualizing the research question via this method will also help you be parsimonious with the number of covariates to consider and include in statistical analyses. 20 A good place to start learning about this visualization method is DAGitty.net . 22
Figure 1: Example of a Directed Acyclic Graph (DAG) of an opioid taper protocol and dependency.
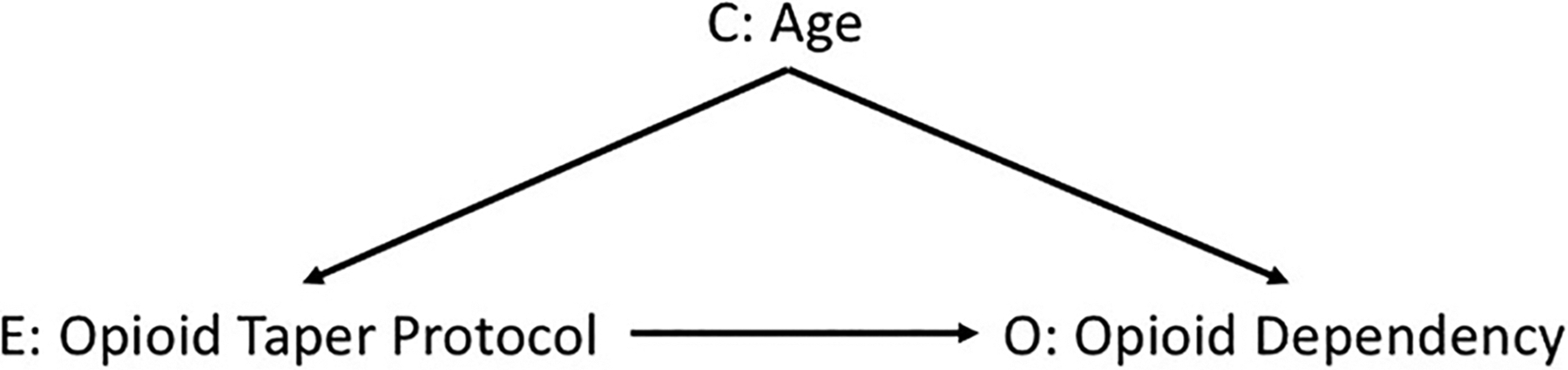
E indicates the exposure variable, O indicates the outcome of interest, and C indicates a confounding variable.
Additionally, even if your associational estimates cannot be interpreted as causal effects, you may be able to perform additional sensitivity or quantitative bias analyses to bolster the causal evidence. For example, suppose you conduct a randomized trial of an experimental opioid taper protocol, but you are only able to follow up 90% of your participants to the trial’s end point. Because the actual effect of the opioid taper depends on the outcomes of the full 100% of participants, you cannot directly interpret your statistical parameter as the estimated causal effect of the taper. However, you could conduct secondary analyses that explore what the statistical parameter would be if everyone who was lost to follow up developed long-term opioid use and what the parameter would be if nobody who was lost to follow up developed long-term opioid use. These analyses would thus place bounds on the impact of your loss to follow-up. This is one example of the broader field of quantitative bias analysis , which is an analytic approach to exploring how much error would need to be present in a study to meaningfully change the appropriate interpretation of findings. 23 , 24
More broadly, if you are careful in how you refer to the association you estimated, your discussion can interpret your results in light of your causal question of interest. For example, in a multisite study evaluating the association between opioid prescriptions after abdominal trauma and ongoing pain at two-week follow-up, you might be concerned that referral patterns affect the severity of abdominal injuries treated at hospitals with different solid organ injury management protocols beyond what can be accounted for statistically using injury severity scores. You should then report the association you observe, but also remind readers whether or not your association is consistent with your hypothesis that opioid prescriptions are not associated with pain two weeks after injury. Many analyses can support this approach, including instrumental variables, inverse probability weighting, or targeted maximum likelihood estimation, among others. 25 – 27
What do you do if you are not confident your analysis can produce an estimate of a causal effect?
When you have a causal research question, it is appropriate to use causal language throughout your writing to describe your question and underlying hypothesis. You would like to know whether your exposure causes your outcome. However, when you cannot interpret your estimates as causal effects, you should ensure that the language you use to report your findings does not imply that your study produced such an estimate. 28
For instance, the word “effect” is used to denote the causal impact of an exposure on an outcome; if your statistical parameter cannot be interpreted as a causal effect, you can still describe what you actually estimated, which was “the association between” your exposure and outcome. Table 2 contains some easy substitutions for causal language, which can be applied to your results and discussion sections.
Language substitutions for causal/non-causal research
In short, it is important to be precise about both the question you would like to know the answer to (e.g. will prescribing NSAIDs rather than opioids achieve adequate pain control?) and the evidence you actually constructed (e.g. people who received NSAIDs reported adequate pain control and fewer side effects than people who received opioids, even after statistical control for injury type and age).
Causality is at the heart of clinical decision-making, yet formal causal evidence is frequently unavailable to contribute to these decisions. A clinical researcher filling gaps in the evidence typically seeks an answer to a causal question. In practice, that clinician might be unable to conduct an RCT due to resource, ethical, or logistic barriers. Yet any clinical evidence can be useful when it comprises the best available answer to the question, with precision, accuracy, and acknowledgment of limitations. An understanding of the causal assumptions can help identify and articulate these limitations. When possible, partnering early in the research process with collaborators trained in study design can help develop appropriate research designs, and ensure planned research activities are designed to allow estimation of the desired parameter.
Acknowledgments:
The authors would like to thank the Harborview Injury Prevention & Research Center as well as Dr. Anjum Hajat, Dr. Ali Rowhani-Rahbar, and Dr. Marco Carone for their input and support in developing this manuscript.
This work did not receive specific funding but authors were supported by the following grants during manuscript preparation: National Center for Advancing Translational Sciences of the National Institutes of Health (TL1 TR002318), National Institute of Child Health and Human Development (T32HD057822, K23HD100566), National Library of Medicine (K99LM012868), National Cancer Institute (T32CA094880, T32CA094061), National Institute of Environmental Health Sciences (T32ES015459), and the Firearm Safety Among Children & Teens Consortium funded by the National Institute for Child Health and Human Development (1R24HD087149).
Role of Funder/Sponsor:
The NIH had no role in the design, writing, or submission of the work. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Conflicts of Interest: The authors have no conflicts of interest to declare.
- 1. Jain MK, Cheung VG, Utz PJ, et al. Saving the Endangered Physician-Scientist - A Plan for Accelerating Medical Breakthroughs. N Engl J Med. 2019;381(5):399–402. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Rahman S, Majumder MA, Shaban SF, et al. Physician participation in clinical research and trials: issues and approaches. Advances in medical education and practice. 2011;2:85–93. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Paget SP, Caldwell PH, Murphy J, et al. Moving beyond ‘not enough time’: factors influencing paediatric clinicians’ participation in research. Internal medicine journal. 2017;47(3):299–306. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Stone C, Dogbey GY, Klenzak S, et al. Contemporary global perspectives of medical students on research during undergraduate medical education: a systematic literature review. Medical education online. 2018;23(1):1537430. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Sumi E, Murayama T, Yokode M. A survey of attitudes toward clinical research among physicians at Kyoto University Hospital. BMC medical education. 2009;9:75. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Yeh HC, Bertram A, Brancati FL, et al. Perceptions of division directors in general internal medicine about the importance of and support for scholarly work done by clinician-educators. Academic medicine : journal of the Association of American Medical Colleges. 2015;90(2):203–208. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. West CP, Ficalora RD. Clinician attitudes toward biostatistics. Mayo Clinic proceedings. 2007;82(8):939–943. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Kosik RO, Tran DT, Fan AP, et al. Physician Scientist Training in the United States: A Survey of the Current Literature. Evaluation & the health professions. 2016;39(1):3–20. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Scriven M A Summative Evaluation of RCT Methodology: & An Alternative Approach to Causal Research. Journal of MultiDisciplinary Evaluation. 2008;5(9):11–24. [ Google Scholar ]
- 10. Vandenbroucke JP, Broadbent A, Pearce N. Causality and causal inference in epidemiology: the need for a pluralistic approach. International journal of epidemiology. 2016;45(6):1776–1786. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 11. Cartwright N Are RCTs the Gold Standard? BioSocieties. 2007;2(1):11–20. [ Google Scholar ]
- 12. Sackett DL. Evidence-based medicine. Seminars in perinatology. 1997;21(1):3–5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Kaufman JS. There is no virtue in vagueness: Comment on: Causal Identification: A Charge of Epidemiology in Danger of Marginalization by Sharon Schwartz, Nicolle M. Gatto, and Ulka B. Campbell. Annals of epidemiology. 2016;26(10):683–684. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Hernán MA, Robins JM. Estimating causal effects from epidemiological data. Journal of epidemiology and community health. 2006;60(7):578–586. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 15. Petersen ML, van der Laan MJ. Causal models and learning from data: integrating causal modeling and statistical estimation. Epidemiology (Cambridge, Mass). 2014;25(3):418–426. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Cole SR, Frangakis CE. The consistency statement in causal inference: a definition or an assumption? Epidemiology (Cambridge, Mass). 2009;20(1):3–5. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Westreich D, Cole SR. Invited commentary: positivity in practice. American journal of epidemiology. 2010;171(6):674–677; discussion 678–681. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Westreich D, Edwards JK, Lesko CR, et al. Target Validity and the Hierarchy of Study Designs. American journal of epidemiology. 2019;188(2):438–443. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Deaton A, Cartwright N. Understanding and misunderstanding randomized controlled trials. Soc Sci Med. 2018;210:2–21. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Pearce N, Lawlor DA. Causal inference-so much more than statistics. International journal of epidemiology. 2016;45(6):1895–1903. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. NIDA. What is the scope of prescription drug misuse in the United States? National Institute on Drug Abuse website. January 26, 2022. https://nida.nih.gov/publications/research-reports/misuse-prescription-drugs/what-scope-prescription-drug-misuse . Accessed March 2, 2022.
- 22. Textor J, van der Zander B, Gilthorpe MS, et al. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. International journal of epidemiology. 2016;45(6):1887–1894. [ DOI ] [ PubMed ] [ Google Scholar ]
- 23. VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Annals of internal medicine. 2017;167(4):268–274. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Lash TL FM, Fink AK. Applying quantitative bias analysis to epidemiologic data Vol 192. New York: Springer; 2009. [ Google Scholar ]
- 25. Hogan JW, Lancaster T. Instrumental variables and inverse probability weighting for causal inference from longitudinal observational studies. Statistical methods in medical research. 2004;13(1):17–48. [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Ohlsson H, Kendler KS. Applying Causal Inference Methods in Psychiatric Epidemiology: A Review. JAMA psychiatry. 2020;77(6):637–644. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Schuler MS, Rose S. Targeted Maximum Likelihood Estimation for Causal Inference in Observational Studies. American journal of epidemiology. 2017;185(1):65–73. [ DOI ] [ PubMed ] [ Google Scholar ]
- 28. Petitti DB. Associations are not effects. American journal of epidemiology. 1991;133(2):101–102. [ DOI ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (102.5 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Root out friction in every digital experience, super-charge conversion rates, and optimize digital self-service
Uncover insights from any interaction, deliver AI-powered agent coaching, and reduce cost to serve
Increase revenue and loyalty with real-time insights and recommendations delivered to teams on the ground
Know how your people feel and empower managers to improve employee engagement, productivity, and retention
Take action in the moments that matter most along the employee journey and drive bottom line growth
Whatever they’re saying, wherever they’re saying it, know exactly what’s going on with your people
Get faster, richer insights with qual and quant tools that make powerful market research available to everyone
Run concept tests, pricing studies, prototyping + more with fast, powerful studies designed by UX research experts
Track your brand performance 24/7 and act quickly to respond to opportunities and challenges in your market
Explore the platform powering Experience Management
- Free Account
- Product Demos
- For Digital
- For Customer Care
- For Human Resources
- For Researchers
- Financial Services
- All Industries
Popular Use Cases
- Customer Experience
- Employee Experience
- Net Promoter Score
- Voice of Customer
- Customer Success Hub
- Product Documentation
- Training & Certification
- XM Institute
- Popular Resources
- Customer Stories
- Artificial Intelligence
Market Research
- Partnerships
- Marketplace
The annual gathering of the experience leaders at the world’s iconic brands building breakthrough business results, live in Salt Lake City.
- English/AU & NZ
- Español/Europa
- Español/América Latina
- Português Brasileiro
- REQUEST DEMO
- Experience Management
- Causal Research
Try Qualtrics for free
Causal research: definition, examples and how to use it.
16 min read Causal research enables market researchers to predict hypothetical occurrences & outcomes while improving existing strategies. Discover how this research can decrease employee retention & increase customer success for your business.
What is causal research?
Causal research, also known as explanatory research or causal-comparative research, identifies the extent and nature of cause-and-effect relationships between two or more variables.
It’s often used by companies to determine the impact of changes in products, features, or services process on critical company metrics. Some examples:
- How does rebranding of a product influence intent to purchase?
- How would expansion to a new market segment affect projected sales?
- What would be the impact of a price increase or decrease on customer loyalty?
To maintain the accuracy of causal research, ‘confounding variables’ or influences — e.g. those that could distort the results — are controlled. This is done either by keeping them constant in the creation of data, or by using statistical methods. These variables are identified before the start of the research experiment.
As well as the above, research teams will outline several other variables and principles in causal research:
- Independent variables
The variables that may cause direct changes in another variable. For example, the effect of truancy on a student’s grade point average. The independent variable is therefore class attendance.
- Control variables
These are the components that remain unchanged during the experiment so researchers can better understand what conditions create a cause-and-effect relationship.
This describes the cause-and-effect relationship. When researchers find causation (or the cause), they’ve conducted all the processes necessary to prove it exists.
- Correlation
Any relationship between two variables in the experiment. It’s important to note that correlation doesn’t automatically mean causation. Researchers will typically establish correlation before proving cause-and-effect.
- Experimental design
Researchers use experimental design to define the parameters of the experiment — e.g. categorizing participants into different groups.
- Dependent variables
These are measurable variables that may change or are influenced by the independent variable. For example, in an experiment about whether or not terrain influences running speed, your dependent variable is the terrain.
Why is causal research useful?
It’s useful because it enables market researchers to predict hypothetical occurrences and outcomes while improving existing strategies. This allows businesses to create plans that benefit the company. It’s also a great research method because researchers can immediately see how variables affect each other and under what circumstances.
Also, once the first experiment has been completed, researchers can use the learnings from the analysis to repeat the experiment or apply the findings to other scenarios. Because of this, it’s widely used to help understand the impact of changes in internal or commercial strategy to the business bottom line.
Some examples include:
- Understanding how overall training levels are improved by introducing new courses
- Examining which variations in wording make potential customers more interested in buying a product
- Testing a market’s response to a brand-new line of products and/or services
So, how does causal research compare and differ from other research types?
Well, there are a few research types that are used to find answers to some of the examples above:
1. Exploratory research
As its name suggests, exploratory research involves assessing a situation (or situations) where the problem isn’t clear. Through this approach, researchers can test different avenues and ideas to establish facts and gain a better understanding.
Researchers can also use it to first navigate a topic and identify which variables are important. Because no area is off-limits, the research is flexible and adapts to the investigations as it progresses.
Finally, this approach is unstructured and often involves gathering qualitative data, giving the researcher freedom to progress the research according to their thoughts and assessment. However, this may make results susceptible to researcher bias and may limit the extent to which a topic is explored.
2. Descriptive research
Descriptive research is all about describing the characteristics of the population, phenomenon or scenario studied. It focuses more on the “what” of the research subject than the “why”.
For example, a clothing brand wants to understand the fashion purchasing trends amongst buyers in California — so they conduct a demographic survey of the region, gather population data and then run descriptive research. The study will help them to uncover purchasing patterns amongst fashion buyers in California, but not necessarily why those patterns exist.
As the research happens in a natural setting, variables can cross-contaminate other variables, making it harder to isolate cause and effect relationships. Therefore, further research will be required if more causal information is needed.
Get started on your market research journey with Strategic Research
How is causal research different from the other two methods above?
Well, causal research looks at what variables are involved in a problem and ‘why’ they act a certain way. As the experiment takes place in a controlled setting (thanks to controlled variables) it’s easier to identify cause-and-effect amongst variables.
Furthermore, researchers can carry out causal research at any stage in the process, though it’s usually carried out in the later stages once more is known about a particular topic or situation.
Finally, compared to the other two methods, causal research is more structured, and researchers can combine it with exploratory and descriptive research to assist with research goals.
Summary of three research types
What are the advantages of causal research?
- Improve experiences
By understanding which variables have positive impacts on target variables (like sales revenue or customer loyalty), businesses can improve their processes, return on investment, and the experiences they offer customers and employees.
- Help companies improve internally
By conducting causal research, management can make informed decisions about improving their employee experience and internal operations. For example, understanding which variables led to an increase in staff turnover.
- Repeat experiments to enhance reliability and accuracy of results
When variables are identified, researchers can replicate cause-and-effect with ease, providing them with reliable data and results to draw insights from.
- Test out new theories or ideas
If causal research is able to pinpoint the exact outcome of mixing together different variables, research teams have the ability to test out ideas in the same way to create viable proof of concepts.
- Fix issues quickly
Once an undesirable effect’s cause is identified, researchers and management can take action to reduce the impact of it or remove it entirely, resulting in better outcomes.
What are the disadvantages of causal research?
- Provides information to competitors
If you plan to publish your research, it provides information about your plans to your competitors. For example, they might use your research outcomes to identify what you are up to and enter the market before you.
- Difficult to administer
Causal research is often difficult to administer because it’s not possible to control the effects of extraneous variables.
- Time and money constraints
Budgetary and time constraints can make this type of research expensive to conduct and repeat. Also, if an initial attempt doesn’t provide a cause and effect relationship, the ROI is wasted and could impact the appetite for future repeat experiments.
- Requires additional research to ensure validity
You can’t rely on just the outcomes of causal research as it’s inaccurate. It’s best to conduct other types of research alongside it to confirm its output.
- Trouble establishing cause and effect
Researchers might identify that two variables are connected, but struggle to determine which is the cause and which variable is the effect.
- Risk of contamination
There’s always the risk that people outside your market or area of study could affect the results of your research. For example, if you’re conducting a retail store study, shoppers outside your ‘test parameters’ shop at your store and skew the results.
How can you use causal research effectively?
To better highlight how you can use causal research across functions or markets, here are a few examples:
Market and advertising research
A company might want to know if their new advertising campaign or marketing campaign is having a positive impact. So, their research team can carry out a causal research project to see which variables cause a positive or negative effect on the campaign.
For example, a cold-weather apparel company in a winter ski-resort town may see an increase in sales generated after a targeted campaign to skiers. To see if one caused the other, the research team could set up a duplicate experiment to see if the same campaign would generate sales from non-skiers. If the results reduce or change, then it’s likely that the campaign had a direct effect on skiers to encourage them to purchase products.
Improving customer experiences and loyalty levels
Customers enjoy shopping with brands that align with their own values, and they’re more likely to buy and present the brand positively to other potential shoppers as a result. So, it’s in your best interest to deliver great experiences and retain your customers.
For example, the Harvard Business Review found that an increase in customer retention rates by 5% increased profits by 25% to 95%. But let’s say you want to increase your own, how can you identify which variables contribute to it?Using causal research, you can test hypotheses about which processes, strategies or changes influence customer retention. For example, is it the streamlined checkout? What about the personalized product suggestions? Or maybe it was a new solution that solved their problem? Causal research will help you find out.
Improving problematic employee turnover rates
If your company has a high attrition rate, causal research can help you narrow down the variables or reasons which have the greatest impact on people leaving. This allows you to prioritize your efforts on tackling the issues in the right order, for the best positive outcomes.
For example, through causal research, you might find that employee dissatisfaction due to a lack of communication and transparency from upper management leads to poor morale, which in turn influences employee retention.
To rectify the problem, you could implement a routine feedback loop or session that enables your people to talk to your company’s C-level executives so that they feel heard and understood.
How to conduct causal research first steps to getting started are:
1. Define the purpose of your research
What questions do you have? What do you expect to come out of your research? Think about which variables you need to test out the theory.
2. Pick a random sampling if participants are needed
Using a technology solution to support your sampling, like a database, can help you define who you want your target audience to be, and how random or representative they should be.
3. Set up the controlled experiment
Once you’ve defined which variables you’d like to measure to see if they interact, think about how best to set up the experiment. This could be in-person or in-house via interviews, or it could be done remotely using online surveys.
4. Carry out the experiment
Make sure to keep all irrelevant variables the same, and only change the causal variable (the one that causes the effect) to gather the correct data. Depending on your method, you could be collecting qualitative or quantitative data, so make sure you note your findings across each regularly.
5. Analyze your findings
Either manually or using technology, analyze your data to see if any trends, patterns or correlations emerge. By looking at the data, you’ll be able to see what changes you might need to do next time, or if there are questions that require further research.
6. Verify your findings
Your first attempt gives you the baseline figures to compare the new results to. You can then run another experiment to verify your findings.
7. Do follow-up or supplemental research
You can supplement your original findings by carrying out research that goes deeper into causes or explores the topic in more detail. One of the best ways to do this is to use a survey. See ‘Use surveys to help your experiment’.
Identifying causal relationships between variables
To verify if a causal relationship exists, you have to satisfy the following criteria:
- Nonspurious association
A clear correlation exists between one cause and the effect. In other words, no ‘third’ that relates to both (cause and effect) should exist.
- Temporal sequence
The cause occurs before the effect. For example, increased ad spend on product marketing would contribute to higher product sales.
- Concomitant variation
The variation between the two variables is systematic. For example, if a company doesn’t change its IT policies and technology stack, then changes in employee productivity were not caused by IT policies or technology.
How surveys help your causal research experiments?
There are some surveys that are perfect for assisting researchers with understanding cause and effect. These include:
- Employee Satisfaction Survey – An introductory employee satisfaction survey that provides you with an overview of your current employee experience.
- Manager Feedback Survey – An introductory manager feedback survey geared toward improving your skills as a leader with valuable feedback from your team.
- Net Promoter Score (NPS) Survey – Measure customer loyalty and understand how your customers feel about your product or service using one of the world’s best-recognized metrics.
- Employee Engagement Survey – An entry-level employee engagement survey that provides you with an overview of your current employee experience.
- Customer Satisfaction Survey – Evaluate how satisfied your customers are with your company, including the products and services you provide and how they are treated when they buy from you.
- Employee Exit Interview Survey – Understand why your employees are leaving and how they’ll speak about your company once they’re gone.
- Product Research Survey – Evaluate your consumers’ reaction to a new product or product feature across every stage of the product development journey.
- Brand Awareness Survey – Track the level of brand awareness in your target market, including current and potential future customers.
- Online Purchase Feedback Survey – Find out how well your online shopping experience performs against customer needs and expectations.
That covers the fundamentals of causal research and should give you a foundation for ongoing studies to assess opportunities, problems, and risks across your market, product, customer, and employee segments.
If you want to transform your research, empower your teams and get insights on tap to get ahead of the competition, maybe it’s time to leverage Qualtrics CoreXM.
Qualtrics CoreXM provides a single platform for data collection and analysis across every part of your business — from customer feedback to product concept testing. What’s more, you can integrate it with your existing tools and services thanks to a flexible API.
Qualtrics CoreXM offers you as much or as little power and complexity as you need, so whether you’re running simple surveys or more advanced forms of research, it can deliver every time.
Get started on your market research journey with CoreXM
Related resources
Mixed methods research 17 min read, market intelligence 10 min read, marketing insights 11 min read, ethnographic research 11 min read, qualitative vs quantitative research 13 min read, primary vs secondary research 14 min read, business research methods 12 min read, request demo.
Ready to learn more about Qualtrics?
Causal Research: Definition, Design, Tips, Examples
Appinio Research · 21.02.2024 · 33min read

Ever wondered why certain events lead to specific outcomes? Understanding causality—the relationship between cause and effect—is crucial for unraveling the mysteries of the world around us. In this guide on causal research, we delve into the methods, techniques, and principles behind identifying and establishing cause-and-effect relationships between variables. Whether you're a seasoned researcher or new to the field, this guide will equip you with the knowledge and tools to conduct rigorous causal research and draw meaningful conclusions that can inform decision-making and drive positive change.
What is Causal Research?
Causal research is a methodological approach used in scientific inquiry to investigate cause-and-effect relationships between variables. Unlike correlational or descriptive research, which merely examine associations or describe phenomena, causal research aims to determine whether changes in one variable cause changes in another variable.

Importance of Causal Research
Understanding the importance of causal research is crucial for appreciating its role in advancing knowledge and informing decision-making across various fields. Here are key reasons why causal research is significant:
- Establishing Causality: Causal research enables researchers to determine whether changes in one variable directly cause changes in another variable. This helps identify effective interventions, predict outcomes, and inform evidence-based practices.
- Guiding Policy and Practice: By identifying causal relationships, causal research provides empirical evidence to support policy decisions, program interventions, and business strategies. Decision-makers can use causal findings to allocate resources effectively and address societal challenges.
- Informing Predictive Modeling : Causal research contributes to the development of predictive models by elucidating causal mechanisms underlying observed phenomena. Predictive models based on causal relationships can accurately forecast future outcomes and trends.
- Advancing Scientific Knowledge: Causal research contributes to the cumulative body of scientific knowledge by testing hypotheses, refining theories, and uncovering underlying mechanisms of phenomena. It fosters a deeper understanding of complex systems and phenomena.
- Mitigating Confounding Factors: Understanding causal relationships allows researchers to control for confounding variables and reduce bias in their studies. By isolating the effects of specific variables, researchers can draw more valid and reliable conclusions.
Causal Research Distinction from Other Research
Understanding the distinctions between causal research and other types of research methodologies is essential for researchers to choose the most appropriate approach for their study objectives. Let's explore the differences and similarities between causal research and descriptive, exploratory, and correlational research methodologies .
Descriptive vs. Causal Research
Descriptive research focuses on describing characteristics, behaviors, or phenomena without manipulating variables or establishing causal relationships. It provides a snapshot of the current state of affairs but does not attempt to explain why certain phenomena occur.
Causal research , on the other hand, seeks to identify cause-and-effect relationships between variables by systematically manipulating independent variables and observing their effects on dependent variables. Unlike descriptive research, causal research aims to determine whether changes in one variable directly cause changes in another variable.
Similarities:
- Both descriptive and causal research involve empirical observation and data collection.
- Both types of research contribute to the scientific understanding of phenomena, albeit through different approaches.
Differences:
- Descriptive research focuses on describing phenomena, while causal research aims to explain why phenomena occur by identifying causal relationships.
- Descriptive research typically uses observational methods, while causal research often involves experimental designs or causal inference techniques to establish causality.
Exploratory vs. Causal Research
Exploratory research aims to explore new topics, generate hypotheses, or gain initial insights into phenomena. It is often conducted when little is known about a subject and seeks to generate ideas for further investigation.
Causal research , on the other hand, is concerned with testing hypotheses and establishing cause-and-effect relationships between variables. It builds on existing knowledge and seeks to confirm or refute causal hypotheses through systematic investigation.
- Both exploratory and causal research contribute to the generation of knowledge and theory development.
- Both types of research involve systematic inquiry and data analysis to answer research questions.
- Exploratory research focuses on generating hypotheses and exploring new areas of inquiry, while causal research aims to test hypotheses and establish causal relationships.
- Exploratory research is more flexible and open-ended, while causal research follows a more structured and hypothesis-driven approach.
Correlational vs. Causal Research
Correlational research examines the relationship between variables without implying causation. It identifies patterns of association or co-occurrence between variables but does not establish the direction or causality of the relationship.
Causal research , on the other hand, seeks to establish cause-and-effect relationships between variables by systematically manipulating independent variables and observing their effects on dependent variables. It goes beyond mere association to determine whether changes in one variable directly cause changes in another variable.
- Both correlational and causal research involve analyzing relationships between variables.
- Both types of research contribute to understanding the nature of associations between variables.
- Correlational research focuses on identifying patterns of association, while causal research aims to establish causal relationships.
- Correlational research does not manipulate variables, while causal research involves systematically manipulating independent variables to observe their effects on dependent variables.
How to Formulate Causal Research Hypotheses?
Crafting research questions and hypotheses is the foundational step in any research endeavor. Defining your variables clearly and articulating the causal relationship you aim to investigate is essential. Let's explore this process further.
1. Identify Variables
Identifying variables involves recognizing the key factors you will manipulate or measure in your study. These variables can be classified into independent, dependent, and confounding variables.
- Independent Variable (IV): This is the variable you manipulate or control in your study. It is the presumed cause that you want to test.
- Dependent Variable (DV): The dependent variable is the outcome or response you measure. It is affected by changes in the independent variable.
- Confounding Variables: These are extraneous factors that may influence the relationship between the independent and dependent variables, leading to spurious correlations or erroneous causal inferences. Identifying and controlling for confounding variables is crucial for establishing valid causal relationships.
2. Establish Causality
Establishing causality requires meeting specific criteria outlined by scientific methodology. While correlation between variables may suggest a relationship, it does not imply causation. To establish causality, researchers must demonstrate the following:
- Temporal Precedence: The cause must precede the effect in time. In other words, changes in the independent variable must occur before changes in the dependent variable.
- Covariation of Cause and Effect: Changes in the independent variable should be accompanied by corresponding changes in the dependent variable. This demonstrates a consistent pattern of association between the two variables.
- Elimination of Alternative Explanations: Researchers must rule out other possible explanations for the observed relationship between variables. This involves controlling for confounding variables and conducting rigorous experimental designs to isolate the effects of the independent variable.
3. Write Clear and Testable Hypotheses
Hypotheses serve as tentative explanations for the relationship between variables and provide a framework for empirical testing. A well-formulated hypothesis should be:
- Specific: Clearly state the expected relationship between the independent and dependent variables.
- Testable: The hypothesis should be capable of being empirically tested through observation or experimentation.
- Falsifiable: There should be a possibility of proving the hypothesis false through empirical evidence.
For example, a hypothesis in a study examining the effect of exercise on weight loss could be: "Increasing levels of physical activity (IV) will lead to greater weight loss (DV) among participants (compared to those with lower levels of physical activity)."
By formulating clear hypotheses and operationalizing variables, researchers can systematically investigate causal relationships and contribute to the advancement of scientific knowledge.
Causal Research Design
Designing your research study involves making critical decisions about how you will collect and analyze data to investigate causal relationships.
Experimental vs. Observational Designs
One of the first decisions you'll make when designing a study is whether to employ an experimental or observational design. Each approach has its strengths and limitations, and the choice depends on factors such as the research question, feasibility , and ethical considerations.
- Experimental Design: In experimental designs, researchers manipulate the independent variable and observe its effects on the dependent variable while controlling for confounding variables. Random assignment to experimental conditions allows for causal inferences to be drawn. Example: A study testing the effectiveness of a new teaching method on student performance by randomly assigning students to either the experimental group (receiving the new teaching method) or the control group (receiving the traditional method).
- Observational Design: Observational designs involve observing and measuring variables without intervention. Researchers may still examine relationships between variables but cannot establish causality as definitively as in experimental designs. Example: A study observing the association between socioeconomic status and health outcomes by collecting data on income, education level, and health indicators from a sample of participants.
Control and Randomization
Control and randomization are crucial aspects of experimental design that help ensure the validity of causal inferences.
- Control: Controlling for extraneous variables involves holding constant factors that could influence the dependent variable, except for the independent variable under investigation. This helps isolate the effects of the independent variable. Example: In a medication trial, controlling for factors such as age, gender, and pre-existing health conditions ensures that any observed differences in outcomes can be attributed to the medication rather than other variables.
- Randomization: Random assignment of participants to experimental conditions helps distribute potential confounders evenly across groups, reducing the likelihood of systematic biases and allowing for causal conclusions. Example: Randomly assigning patients to treatment and control groups in a clinical trial ensures that both groups are comparable in terms of baseline characteristics, minimizing the influence of extraneous variables on treatment outcomes.
Internal and External Validity
Two key concepts in research design are internal validity and external validity, which relate to the credibility and generalizability of study findings, respectively.
- Internal Validity: Internal validity refers to the extent to which the observed effects can be attributed to the manipulation of the independent variable rather than confounding factors. Experimental designs typically have higher internal validity due to their control over extraneous variables. Example: A study examining the impact of a training program on employee productivity would have high internal validity if it could confidently attribute changes in productivity to the training intervention.
- External Validity: External validity concerns the extent to which study findings can be generalized to other populations, settings, or contexts. While experimental designs prioritize internal validity, they may sacrifice external validity by using highly controlled conditions that do not reflect real-world scenarios. Example: Findings from a laboratory study on memory retention may have limited external validity if the experimental tasks and conditions differ significantly from real-life learning environments.
Types of Experimental Designs
Several types of experimental designs are commonly used in causal research, each with its own strengths and applications.
- Randomized Control Trials (RCTs): RCTs are considered the gold standard for assessing causality in research. Participants are randomly assigned to experimental and control groups, allowing researchers to make causal inferences. Example: A pharmaceutical company testing a new drug's efficacy would use an RCT to compare outcomes between participants receiving the drug and those receiving a placebo.
- Quasi-Experimental Designs: Quasi-experimental designs lack random assignment but still attempt to establish causality by controlling for confounding variables through design or statistical analysis . Example: A study evaluating the effectiveness of a smoking cessation program might compare outcomes between participants who voluntarily enroll in the program and a matched control group of non-enrollees.
By carefully selecting an appropriate research design and addressing considerations such as control, randomization, and validity, researchers can conduct studies that yield credible evidence of causal relationships and contribute valuable insights to their field of inquiry.
Causal Research Data Collection
Collecting data is a critical step in any research study, and the quality of the data directly impacts the validity and reliability of your findings.
Choosing Measurement Instruments
Selecting appropriate measurement instruments is essential for accurately capturing the variables of interest in your study. The choice of measurement instrument depends on factors such as the nature of the variables, the target population , and the research objectives.
- Surveys : Surveys are commonly used to collect self-reported data on attitudes, opinions, behaviors, and demographics . They can be administered through various methods, including paper-and-pencil surveys, online surveys, and telephone interviews.
- Observations: Observational methods involve systematically recording behaviors, events, or phenomena as they occur in natural settings. Observations can be structured (following a predetermined checklist) or unstructured (allowing for flexible data collection).
- Psychological Tests: Psychological tests are standardized instruments designed to measure specific psychological constructs, such as intelligence, personality traits, or emotional functioning. These tests often have established reliability and validity.
- Physiological Measures: Physiological measures, such as heart rate, blood pressure, or brain activity, provide objective data on bodily processes. They are commonly used in health-related research but require specialized equipment and expertise.
- Existing Databases: Researchers may also utilize existing datasets, such as government surveys, public health records, or organizational databases, to answer research questions. Secondary data analysis can be cost-effective and time-saving but may be limited by the availability and quality of data.
Ensuring accurate data collection is the cornerstone of any successful research endeavor. With the right tools in place, you can unlock invaluable insights to drive your causal research forward. From surveys to tests, each instrument offers a unique lens through which to explore your variables of interest.
At Appinio , we understand the importance of robust data collection methods in informing impactful decisions. Let us empower your research journey with our intuitive platform, where you can effortlessly gather real-time consumer insights to fuel your next breakthrough. Ready to take your research to the next level? Book a demo today and see how Appinio can revolutionize your approach to data collection!
Book a Demo
Sampling Techniques
Sampling involves selecting a subset of individuals or units from a larger population to participate in the study. The goal of sampling is to obtain a representative sample that accurately reflects the characteristics of the population of interest.
- Probability Sampling: Probability sampling methods involve randomly selecting participants from the population, ensuring that each member of the population has an equal chance of being included in the sample. Common probability sampling techniques include simple random sampling , stratified sampling, and cluster sampling .
- Non-Probability Sampling: Non-probability sampling methods do not involve random selection and may introduce biases into the sample. Examples of non-probability sampling techniques include convenience sampling, purposive sampling, and snowball sampling.
The choice of sampling technique depends on factors such as the research objectives, population characteristics, resources available, and practical constraints. Researchers should strive to minimize sampling bias and maximize the representativeness of the sample to enhance the generalizability of their findings.
Ethical Considerations
Ethical considerations are paramount in research and involve ensuring the rights, dignity, and well-being of research participants. Researchers must adhere to ethical principles and guidelines established by professional associations and institutional review boards (IRBs).
- Informed Consent: Participants should be fully informed about the nature and purpose of the study, potential risks and benefits, their rights as participants, and any confidentiality measures in place. Informed consent should be obtained voluntarily and without coercion.
- Privacy and Confidentiality: Researchers should take steps to protect the privacy and confidentiality of participants' personal information. This may involve anonymizing data, securing data storage, and limiting access to identifiable information.
- Minimizing Harm: Researchers should mitigate any potential physical, psychological, or social harm to participants. This may involve conducting risk assessments, providing appropriate support services, and debriefing participants after the study.
- Respect for Participants: Researchers should respect participants' autonomy, diversity, and cultural values. They should seek to foster a trusting and respectful relationship with participants throughout the research process.
- Publication and Dissemination: Researchers have a responsibility to accurately report their findings and acknowledge contributions from participants and collaborators. They should adhere to principles of academic integrity and transparency in disseminating research results.
By addressing ethical considerations in research design and conduct, researchers can uphold the integrity of their work, maintain trust with participants and the broader community, and contribute to the responsible advancement of knowledge in their field.
Causal Research Data Analysis
Once data is collected, it must be analyzed to draw meaningful conclusions and assess causal relationships.
Causal Inference Methods
Causal inference methods are statistical techniques used to identify and quantify causal relationships between variables in observational data. While experimental designs provide the most robust evidence for causality, observational studies often require more sophisticated methods to account for confounding factors.
- Difference-in-Differences (DiD): DiD compares changes in outcomes before and after an intervention between a treatment group and a control group, controlling for pre-existing trends. It estimates the average treatment effect by differencing the changes in outcomes between the two groups over time.
- Instrumental Variables (IV): IV analysis relies on instrumental variables—variables that affect the treatment variable but not the outcome—to estimate causal effects in the presence of endogeneity. IVs should be correlated with the treatment but uncorrelated with the error term in the outcome equation.
- Regression Discontinuity (RD): RD designs exploit naturally occurring thresholds or cutoff points to estimate causal effects near the threshold. Participants just above and below the threshold are compared, assuming that they are similar except for their proximity to the threshold.
- Propensity Score Matching (PSM): PSM matches individuals or units based on their propensity scores—the likelihood of receiving the treatment—creating comparable groups with similar observed characteristics. Matching reduces selection bias and allows for causal inference in observational studies.
Assessing Causality Strength
Assessing the strength of causality involves determining the magnitude and direction of causal effects between variables. While statistical significance indicates whether an observed relationship is unlikely to occur by chance, it does not necessarily imply a strong or meaningful effect.
- Effect Size: Effect size measures the magnitude of the relationship between variables, providing information about the practical significance of the results. Standard effect size measures include Cohen's d for mean differences and odds ratios for categorical outcomes.
- Confidence Intervals: Confidence intervals provide a range of values within which the actual effect size is likely to lie with a certain degree of certainty. Narrow confidence intervals indicate greater precision in estimating the true effect size.
- Practical Significance: Practical significance considers whether the observed effect is meaningful or relevant in real-world terms. Researchers should interpret results in the context of their field and the implications for stakeholders.
Handling Confounding Variables
Confounding variables are extraneous factors that may distort the observed relationship between the independent and dependent variables, leading to spurious or biased conclusions. Addressing confounding variables is essential for establishing valid causal inferences.
- Statistical Control: Statistical control involves including confounding variables as covariates in regression models to partially out their effects on the outcome variable. Controlling for confounders reduces bias and strengthens the validity of causal inferences.
- Matching: Matching participants or units based on observed characteristics helps create comparable groups with similar distributions of confounding variables. Matching reduces selection bias and mimics the randomization process in experimental designs.
- Sensitivity Analysis: Sensitivity analysis assesses the robustness of study findings to changes in model specifications or assumptions. By varying analytical choices and examining their impact on results, researchers can identify potential sources of bias and evaluate the stability of causal estimates.
- Subgroup Analysis: Subgroup analysis explores whether the relationship between variables differs across subgroups defined by specific characteristics. Identifying effect modifiers helps understand the conditions under which causal effects may vary.
By employing rigorous causal inference methods, assessing the strength of causality, and addressing confounding variables, researchers can confidently draw valid conclusions about causal relationships in their studies, advancing scientific knowledge and informing evidence-based decision-making.
Causal Research Examples
Examples play a crucial role in understanding the application of causal research methods and their impact across various domains. Let's explore some detailed examples to illustrate how causal research is conducted and its real-world implications:
Example 1: Software as a Service (SaaS) User Retention Analysis
Suppose a SaaS company wants to understand the factors influencing user retention and engagement with their platform. The company conducts a longitudinal observational study, collecting data on user interactions, feature usage, and demographic information over several months.
- Design: The company employs an observational cohort study design, tracking cohorts of users over time to observe changes in retention and engagement metrics. They use analytics tools to collect data on user behavior , such as logins, feature usage, session duration, and customer support interactions.
- Data Collection: Data is collected from the company's platform logs, customer relationship management (CRM) system, and user surveys. Key metrics include user churn rates, active user counts, feature adoption rates, and Net Promoter Scores ( NPS ).
- Analysis: Using statistical techniques like survival analysis and regression modeling, the company identifies factors associated with user retention, such as feature usage patterns, onboarding experiences, customer support interactions, and subscription plan types.
- Findings: The analysis reveals that users who engage with specific features early in their lifecycle have higher retention rates, while those who encounter usability issues or lack personalized onboarding experiences are more likely to churn. The company uses these insights to optimize product features, improve onboarding processes, and enhance customer support strategies to increase user retention and satisfaction.
Example 2: Business Impact of Digital Marketing Campaign
Consider a technology startup launching a digital marketing campaign to promote its new product offering. The company conducts an experimental study to evaluate the effectiveness of different marketing channels in driving website traffic, lead generation, and sales conversions.
- Design: The company implements an A/B testing design, randomly assigning website visitors to different marketing treatment conditions, such as Google Ads, social media ads, email campaigns, or content marketing efforts. They track user interactions and conversion events using web analytics tools and marketing automation platforms.
- Data Collection: Data is collected on website traffic, click-through rates, conversion rates, lead generation, and sales revenue. The company also gathers demographic information and user feedback through surveys and customer interviews to understand the impact of marketing messages and campaign creatives .
- Analysis: Utilizing statistical methods like hypothesis testing and multivariate analysis, the company compares key performance metrics across different marketing channels to assess their effectiveness in driving user engagement and conversion outcomes. They calculate return on investment (ROI) metrics to evaluate the cost-effectiveness of each marketing channel.
- Findings: The analysis reveals that social media ads outperform other marketing channels in generating website traffic and lead conversions, while email campaigns are more effective in nurturing leads and driving sales conversions. Armed with these insights, the company allocates marketing budgets strategically, focusing on channels that yield the highest ROI and adjusting messaging and targeting strategies to optimize campaign performance.
These examples demonstrate the diverse applications of causal research methods in addressing important questions, informing policy decisions, and improving outcomes in various fields. By carefully designing studies, collecting relevant data, employing appropriate analysis techniques, and interpreting findings rigorously, researchers can generate valuable insights into causal relationships and contribute to positive social change.
How to Interpret Causal Research Results?
Interpreting and reporting research findings is a crucial step in the scientific process, ensuring that results are accurately communicated and understood by stakeholders.
Interpreting Statistical Significance
Statistical significance indicates whether the observed results are unlikely to occur by chance alone, but it does not necessarily imply practical or substantive importance. Interpreting statistical significance involves understanding the meaning of p-values and confidence intervals and considering their implications for the research findings.
- P-values: A p-value represents the probability of obtaining the observed results (or more extreme results) if the null hypothesis is true. A p-value below a predetermined threshold (typically 0.05) suggests that the observed results are statistically significant, indicating that the null hypothesis can be rejected in favor of the alternative hypothesis.
- Confidence Intervals: Confidence intervals provide a range of values within which the true population parameter is likely to lie with a certain degree of confidence (e.g., 95%). If the confidence interval does not include the null value, it suggests that the observed effect is statistically significant at the specified confidence level.
Interpreting statistical significance requires considering factors such as sample size, effect size, and the practical relevance of the results rather than relying solely on p-values to draw conclusions.
Discussing Practical Significance
While statistical significance indicates whether an effect exists, practical significance evaluates the magnitude and meaningfulness of the effect in real-world terms. Discussing practical significance involves considering the relevance of the results to stakeholders and assessing their impact on decision-making and practice.
- Effect Size: Effect size measures the magnitude of the observed effect, providing information about its practical importance. Researchers should interpret effect sizes in the context of their field and the scale of measurement (e.g., small, medium, or large effect sizes).
- Contextual Relevance: Consider the implications of the results for stakeholders, policymakers, and practitioners. Are the observed effects meaningful in the context of existing knowledge, theory, or practical applications? How do the findings contribute to addressing real-world problems or informing decision-making?
Discussing practical significance helps contextualize research findings and guide their interpretation and application in practice, beyond statistical significance alone.
Addressing Limitations and Assumptions
No study is without limitations, and researchers should transparently acknowledge and address potential biases, constraints, and uncertainties in their research design and findings.
- Methodological Limitations: Identify any limitations in study design, data collection, or analysis that may affect the validity or generalizability of the results. For example, sampling biases , measurement errors, or confounding variables.
- Assumptions: Discuss any assumptions made in the research process and their implications for the interpretation of results. Assumptions may relate to statistical models, causal inference methods, or theoretical frameworks underlying the study.
- Alternative Explanations: Consider alternative explanations for the observed results and discuss their potential impact on the validity of causal inferences. How robust are the findings to different interpretations or competing hypotheses?
Addressing limitations and assumptions demonstrates transparency and rigor in the research process, allowing readers to critically evaluate the validity and reliability of the findings.
Communicating Findings Clearly
Effectively communicating research findings is essential for disseminating knowledge, informing decision-making, and fostering collaboration and dialogue within the scientific community.
- Clarity and Accessibility: Present findings in a clear, concise, and accessible manner, using plain language and avoiding jargon or technical terminology. Organize information logically and use visual aids (e.g., tables, charts, graphs) to enhance understanding.
- Contextualization: Provide context for the results by summarizing key findings, highlighting their significance, and relating them to existing literature or theoretical frameworks. Discuss the implications of the findings for theory, practice, and future research directions.
- Transparency: Be transparent about the research process, including data collection procedures, analytical methods, and any limitations or uncertainties associated with the findings. Clearly state any conflicts of interest or funding sources that may influence interpretation.
By communicating findings clearly and transparently, researchers can facilitate knowledge exchange, foster trust and credibility, and contribute to evidence-based decision-making.
Causal Research Tips
When conducting causal research, it's essential to approach your study with careful planning, attention to detail, and methodological rigor. Here are some tips to help you navigate the complexities of causal research effectively:
- Define Clear Research Questions: Start by clearly defining your research questions and hypotheses. Articulate the causal relationship you aim to investigate and identify the variables involved.
- Consider Alternative Explanations: Be mindful of potential confounding variables and alternative explanations for the observed relationships. Take steps to control for confounders and address alternative hypotheses in your analysis.
- Prioritize Internal Validity: While external validity is important for generalizability, prioritize internal validity in your study design to ensure that observed effects can be attributed to the manipulation of the independent variable.
- Use Randomization When Possible: If feasible, employ randomization in experimental designs to distribute potential confounders evenly across experimental conditions and enhance the validity of causal inferences.
- Be Transparent About Methods: Provide detailed descriptions of your research methods, including data collection procedures, analytical techniques, and any assumptions or limitations associated with your study.
- Utilize Multiple Methods: Consider using a combination of experimental and observational methods to triangulate findings and strengthen the validity of causal inferences.
- Be Mindful of Sample Size: Ensure that your sample size is adequate to detect meaningful effects and minimize the risk of Type I and Type II errors. Conduct power analyses to determine the sample size needed to achieve sufficient statistical power.
- Validate Measurement Instruments: Validate your measurement instruments to ensure that they are reliable and valid for assessing the variables of interest in your study. Pilot test your instruments if necessary.
- Seek Feedback from Peers: Collaborate with colleagues or seek feedback from peer reviewers to solicit constructive criticism and improve the quality of your research design and analysis.
Conclusion for Causal Research
Mastering causal research empowers researchers to unlock the secrets of cause and effect, shedding light on the intricate relationships between variables in diverse fields. By employing rigorous methods such as experimental designs, causal inference techniques, and careful data analysis, you can uncover causal mechanisms, predict outcomes, and inform evidence-based practices. Through the lens of causal research, complex phenomena become more understandable, and interventions become more effective in addressing societal challenges and driving progress. In a world where understanding the reasons behind events is paramount, causal research serves as a beacon of clarity and insight. Armed with the knowledge and techniques outlined in this guide, you can navigate the complexities of causality with confidence, advancing scientific knowledge, guiding policy decisions, and ultimately making meaningful contributions to our understanding of the world.
How to Conduct Causal Research in Minutes?
Introducing Appinio , your gateway to lightning-fast causal research. As a real-time market research platform, we're revolutionizing how companies gain consumer insights to drive data-driven decisions. With Appinio, conducting your own market research is not only easy but also thrilling. Experience the excitement of market research with Appinio, where fast, intuitive, and impactful insights are just a click away.
Here's why you'll love Appinio:
- Instant Insights: Say goodbye to waiting days for research results. With our platform, you'll go from questions to insights in minutes, empowering you to make decisions at the speed of business.
- User-Friendly Interface: No need for a research degree here! Our intuitive platform is designed for anyone to use, making complex research tasks simple and accessible.
- Global Reach: Reach your target audience wherever they are. With access to over 90 countries and the ability to define precise target groups from 1200+ characteristics, you'll gather comprehensive data to inform your decisions.
Get free access to the platform!
Join the loop 💌
Be the first to hear about new updates, product news, and data insights. We'll send it all straight to your inbox.
Get the latest market research news straight to your inbox! 💌
Wait, there's more

04.11.2024 | 5min read
Trustly uses Appinio’s insights to revolutionize utility bill payments

19.09.2024 | 9min read
Track Your Customer Retention & Brand Metrics for Post-Holiday Success

16.09.2024 | 10min read
Creative Checkup – Optimize Advertising Slogans & Creatives for ROI

IMAGES
VIDEO