Science Project Ideas
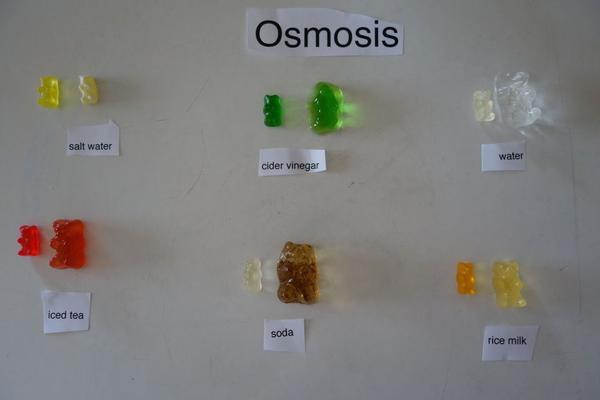

Gummy Bear Experiment
The gummy bear experiment is a fun activity that teaches the basic concept of osmosis to the little ones in an easy manner. They will also be thrilled at the idea that their favorite gummy bears could teach them a lesson or two in science.
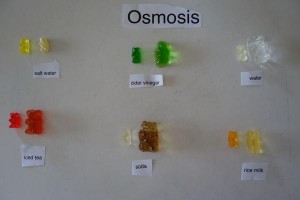
Gummy Bear Science Project Instructions
Hypothesis for growing/shrinking gummy bears.
When a gummy bear is placed in a hypotonic solution (e.g. water) then it will increase in mass and volume. On the other hand, its mass and volume decrease when placed in a hypertonic solution (e.g. salt water). Hypertonic refers to a higher concentration of solutes and hypotonic is just the reverse.
Things Needed
- Physical balance or digital weighing machine
- Sieve, plastic fork or screen
- Graph paper
- Measure the dimensions (length, breadth and height) of the bear with a scale. Measure its mass with the balance.
- Fill the bowl with water.
- Completely immerse the gummy bear in the water.
- Let the bowl sit overnight in a place away from direct sunlight.
- Next day, lift the bear from the water with a plastic fork, sieve or screen.
- Record the dimensions and mass of the bear again.
- Do the same observation each day for a couple of days more.
- Plot a graph with the time in hours along the X-axis (the dependent variable) and the mass or weight of the gummy bear along the Y-axis (independent variable). Check the nature of the graph.
Things You Can Try
- Set up a series of bowls on the table and fill them with different solutions like that of baking soda, vinegar, salt, distilled water, etc. Make similar observations as above for each one of them. Compare your results.
- Also, check if the taste and/or color of the bears have changed.
Gummy Bear Osmosis Video
How does it work.
The ingredients of gummy bears are sugar, water, and gelatin, with little water content. Due to the process of osmosis, i.e., the movement of water molecules through a selectively permeable membrane from an area of high concentration to that of a lower concentration, the bear starts to grow. However, it doesn’t get dissolved as the gelatin is insoluble in water.
On trying out the different ideas, you will find that the degree of expansion of the candy depends on the liquid on which it is kept. However, vinegar, which is actually an acid, can dissolve the candy .
This simple trick can prove to be a cool science fair idea. The kids will be enthralled to display their knowledge to the audience in a fun way.
References:
https://biozone.weebly.com/uploads/2/7/4/2/274298/gummy_bear_osmosis.pdf
https://tinkerlab.com/incredible-growing-gummy-bears/
https://www.childrensmuseum.org/blog/saturday-science-growing-gummy-bears
https://www.homeschool.com/blog/index.php/2014/04/homeschool-science-gummy-bear-osmosis/
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

- 3 gummy bears
- 2 drinking glasses
- Refrigerator
- Something to heat water with (for example a microwave oven or a kettle)

Short explanation
Long explanation.
- How big will a gummy bear be after 1, 2, 4, 6, 12, 24, 48, 72 hours in water?
- Does it matter if you cover the glass or not?
- Is it possible to shrink a swollen gummy bear completely down to its former size again?
- What other things swell in water?
- What other things shrink in sugar water?

Yeast and a balloon

Strawberry DNA
Homemade yogurt

Screaming dry ice

Dry ice in a balloon
Special: Dry ice color change

Dry ice smoking soap bubble snake

Dry ice giant crystal ball bubble

Dry ice in water

Rainbow milk

Gummy bear osmosis

Floating ping pong ball

Rotating Earth
Special: Colored fire

Special: Fire bubbles

Water cycle in a jar

Egg drop challenge
Taking the pulse

Orange candle

Glass bottle xylophone

Warped spacetime

Homemade rainbow

Water implosion

Warm and cold plates

Plastic bag kite

Tamed lightning

Forever boiling bottle

Moon on a pen

Moon in a box

Inexhaustible bottle

Crystal egg geode

Magic ice cut

Leaf pigments chromatography

Heavy smoke

Popsicle stick bridge

Micrometeorites
Special: Fire tornado

Special: Whoosh bottle

Dancing water marbles

Brownian motion

Flying static ring

Water thermometer

String telephone

Special: Dust explosion

Disappearing styrofoam

Special: Burning money

Special: Burning towel

Salt water purifier

Fish dissection

Hovering soap bubble

Homemade sailboat

Water mass meeting

Plastic bag and pencils

Water sucking bottle

Water sucking glass


Mentos and coke
Aristotle's illusion

Spinning spiral snake

Imploding soda can

Carbon dioxide extuingisher

Plastic bag parachute
Dental impression

Impact craters

Rolling static soda can

Static paper ghost

Color changing flower

Upside down glass

Shrinking chip bag

Solar system model

Electric motor
Flashy electric motor
Bouncing soap bubbles

Toilet paper roll maraca

Cloud in a bottle 1

Cloud in a bottle 2

Balloon rocket

Water whistle

Special: Screaming gummy bear
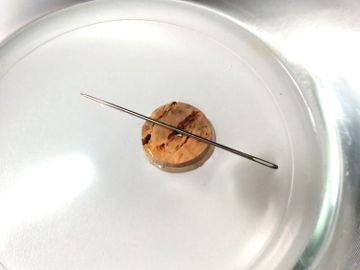
Homemade compass

Trash airplane

Wind-up spinner toy

Tea bag rocket

Balancing soda can

Lung volume test

Fireproof balloon

Baking powder popper

Expanding space

Straw propeller

Wooden cutlery

Levitating match

Human reflexes

Electromagnet

Soil layers

Straw potato

Straw rocket launcher

Traveling flame

Water bowls

Straw duck call
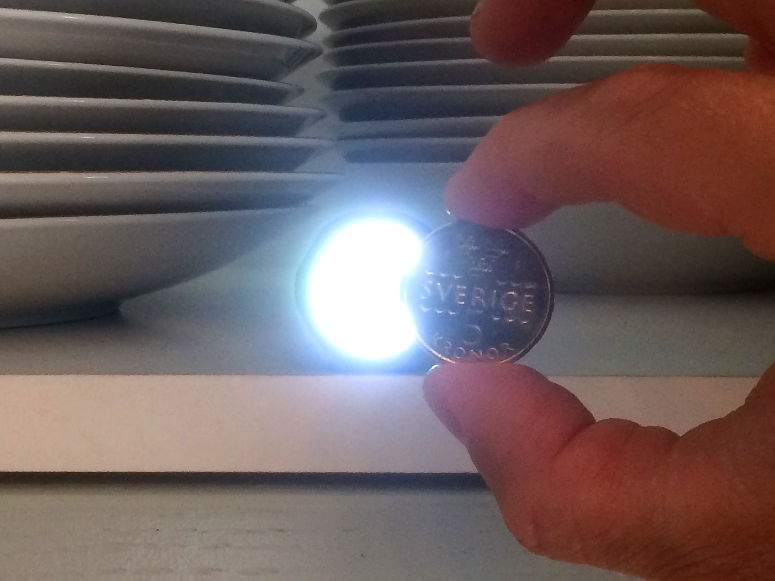
Solar eclipse

Silo of salt

Balloon skewer

Newspaper tower
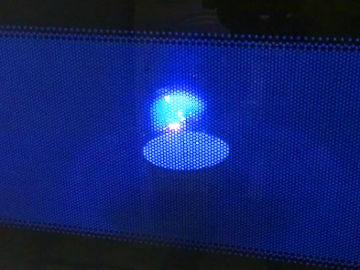
Microwave light bulb

Heavy paper

Rubber chicken bone

Homemade marble run

Drops on a coin

Cartesian diver
Content of website.


Gummy Bear Experiment
Osmosis can be a difficult concept for kids to understand. I’ve always found that visual explanations really hit home with kids and help them to understand. Today we have a growing gummy bear experiment that is a perfect compliment to our Gummy Mummy experiment that explores the science of desiccation and diffusion. Because gummy bears are made of gelatin they will not dissolve in water like other candy will. They will however absorb liquids and change in shape and size. We’ve set up an experiment with four different liquids to see the difference in how the gummy bears are able to absorb each and how they change over the course of the day.
Great Growing Gummies – Gummy Bear Osmosis Experiment
Table of Contents

Disclaimer: This article may contain commission or affiliate links. As an Amazon Influencer I earn from qualifying purchases. Not seeing our videos? Turn off any adblockers to ensure our video feed can be seen. Or visit our YouTube channel to see if the video has been uploaded there. We are slowly uploading our archives. Thanks!
What is Osmosis?
Scientifically, Osmosis is when solvent molecules (usually water) cross a semipermeable membrane from an area of lower solute concentration to an area of higher solute concentration. This creates equilibrium between the solute and solvent, balancing the concentration of solutes on both sides of the membrane. Osmosis is a passive process in that it requires no energy from the cell to occur.
Now – that’s a lot of big words and concepts so let’s break down that vocabulary:
Solvent : substance able to dissolve other substances. Solute : a dissolved substance Membrane : a thin, soft flexible sheet or layer especially of a plant or animal part Semi-Permeable Membrane : a membrane that only allows certain substances to pass through. Concentration : the amount of a component in a given substance. Equilibrium : a state of adjustment between opposing or divergent influences or elements
Gummy Bear Osmosis Lab
Gummy Bears Small Clear Bowls or Jars Water Sparkling Water White Vinegar Oil
I like to start this in the morning so you can check on it throughout the day and see the changes in the gummy bears.
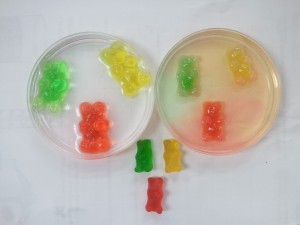
STEP 1: Lay out four bowls on the table and put a gummy bear in each bowl. Then beside each bowl put another gummy bear of the same color so you can compare the two easily over the course of the day.
STEP 2: Measure equal amounts of each of your solvents. We used a quarter of a cup of water, sparkling water, white vinegar and oil and poured them over the gummy bears in the bowl.

STEP 3: This is a great time to have a discussion about osmosis and have your kids make predictions about what they think is going to happen in each bowl and why. What effect might each substance have on the gummy bear? Have the kids write down their predictions.
STEP 4: Set a timer for an hour and let the bears do their thing.
STEP 5: Check back each hour for the rest of the day and write down observations over the course of the day.
Gummy Bear Osmosis Experiment Results
Now the exciting part… the results of our experiment! Let’s take a look at the results individually first.

When gummy bears are soaked in water the bear will swell and grow in size. This is because the water will flow into the gummy bear through its semi-permeable membrane. The sugar molecules try to spread and dissolve but they can’t get out of the gelatin so they expand resulting in the gummy bear expanding.
Sparkling Water

Will have a similar result to water. The only difference is that the addition of carbon dioxide to the water can have an acidic effect on the bears which would cause the outside to soften allowing more water to be able to pass through the bear and it swells up more. You will also be able to observe the carbon dioxide bubble sticking to the outside of the bear.
White Vinegar

White Vinegar will have an acidic reaction with the gummy bear softening the outside of it, however the liquid is not as easily absorbed into the bear as water so the gummy may get softer but will not change in size as much as the bears soaked in water.

Because oil is polar it doesn’t mix well with water or other substances. The oil will have very little effect on the bears and you will not see much change if any at all. This gummy bear will also retain its color the best because the oil isn’t breaking down the bear or being absorbed into it so the structure and color will remain the same.
Comparing the Results
The most fascinating part of this experiment is comparing the results of the different solvents. Set the gummy bears out side by side with their controls so you can visually see the differences.

To get really scientific with your results, which is perfect for your older kids or kids needing more of a challenge, have them weigh and measure the gummies and compare results with the controls and each other.
You can also dissect the gummy bears and view them under a microscope to look for microscopic changes.
Extension Ideas
I think your kids will love this Gummy Bear Lab experiment on Osmosis! Encourage your students to get creative and add other variations like adding things like salt or baking soda to the water to see if it changes the results. Or try other solvents.
Want more osmosis experiments? Try this Rainbow Water Beads Experiment or the Bouncy Egg Experiment which involves a chemical reaction and osmosis.
Want more gummy science? Check out our Gummy Mummies . Or make your own gummies! You can check out these recipes on the site: Valentine’s Day Gummies , Star Wars Gummies , Rainbow Dragon Egg Gummies .
5 Days of Smart STEM Ideas for Kids
Get started in STEM with easy, engaging activities.

IMAGES
VIDEO